Abstract
By disrupting the structure of native ant assemblages, invasive ants can have effects across trophic levels. Most studies to date, however, have focused on the impacts just two species (Linepithema humile and Solenopsis invicta). The impacts of many other invasive ant species on ecological processes in their introduced range are unknown. In this study we tested the hypothesis that the invasive ant Pachycondyla chinensis disrupts ant-seed dispersal mutualisms by displacing native ant species, especially the keystone mutualist Aphaenogaster rudis, while failing to disperse seeds itself. In a paired design we measured the impact of P. chinensis on the native ant-plant seed dispersal mutualism. The number of A. rudis workers was 96% lower in invaded than in intact plots, and the number of seeds removed was 70% lower in these plots. Finally, in invaded plots the abundance of Hexastylis arifolia, a locally abundant myrmecochorous plant, was 50% lower than in plots where P. chinensis was absent. A parsimonious interpretation of our results is that P. chinensis causes precipitous declines in the abundance of A. rudis within invaded communities, thereby disrupting the ant-plant seed dispersal mutualisms and reducing abundances of ant-dispersed plants. In sum, the magnitude of the effects of P. chinensis on seed dispersal is quantitatively similar to that documented for the intensively studied invasive Argentine ant. We suggest that more studies on the impacts of less-studied invasive ant species on seed dispersal mutualisms may increase our knowledge of the effects of these invaders on ecosystem function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Though local communities often include many species, those species are not necessarily equal in their effects on one another. In many cases, a single species can have disproportionate effects on other species and on ecosystem processes (Ellison et al. 2005). Invasive species provide many key examples of the strong effects of single species ramifying through communities and ecosystems. For example, the presence of invasive plant species can alter fire regimes (D’Antonio and Vitousek 1992), nutrient cycling (Vitousek 1990; Tardiff and Stanford 1998), and other ecosystem processes (Levine et al. 2003). The arrival of some invasive species can also disrupt mutualistic interactions such as pollination (Vazquez and Simberloff 2004) or seed dispersal (Traveset and Richardson 2006; Rodriguez-Cabal et al. 2009). Such effects arise, at least in part, because of reductions in the abundance or alterations of the behavior of one or a few native species (Traveset and Richardson 2006; Rodriguez-Cabal et al. 2009).
The disruption of seed dispersal mutualisms can affect the persistence of particular plant species and the structure of plant communities (Howe and Smallwood 1982; Schupp and Fuentes 1995; Wenny and Levey 1998; Jordano and Schupp 2000; Rodriguez-Cabal et al. 2007). Because many seed dispersal mutualisms involve many or at least multiple dispersers, seed dispersal mutualisms may tend to be relatively resilient to changes in the identity of dispersing species (Bascompte et al. 2003, 2006; Vazquez and Aizen 2004; Vazquez et al. 2005). However, a growing number of studies have found that seed dispersal mutualisms are more specialized than they appear (Gilbert 1980; Gove et al. 2007; Manzaneda and Rey 2009; McCoy 2009). In such specialized systems, the impact of invasive species on seed dispersal mutualisms might be especially pronounced.
Invasions by ant species represent an interesting context in which to examine the dependence of seed dispersal on individual species. Studies on Argentine ants (Linepithema humile) and red imported fire ants (Solenopsis invicta) generally indicate strong negative effects on seed dispersal mutualisms. After displacing populations of native seed dispersers, L. humile and S. invicta often fail to disperse seeds effectively (Bond and Slingsby 1984; Carney et al. 2003; Gómez and Oliveras 2003; Gómez et al. 2003; Ness 2004; Rowles and O’Dowd 2009; but see Stuble et al. 2010). The majority of studies of the impacts of invasive ants on seed dispersal have focused on these two ant species (Holway et al. 2002; Ness and Bronstein 2004; Rodriguez-Cabal et al. 2009), though many other introduced ant species are sufficiently abundant to compete with native ants for resources such as seeds (Holway et al. 2002), and even more introduced species could potentially be considered as invasive in the future (McGlynn 1999). Clearly, much remains to be learned about the potential impacts of other invasive ant species on seed dispersal mutualisms.
Pachycondyla chinensis (Emery) (Formicidae: Ponerinae) is an invasive ant whose abundance has recently been shown to be associated with dramatic changes in ant community composition in parts of its introduced range (Guénard and Dunn 2010). P. chinensis reduces the abundances of most native ant species, including the important seed disperser Aphaenogaster rudis (which is part of a species complex, but will be referred to as A. rudis here), while apparently having either no or positive effects on the larger-bodied species in the genera Camponotus and Formica (Guénard and Dunn 2010). In the temperate deciduous forests of eastern North America, A. rudis is responsible for between 48 and 100% of all seed dispersal events, suggesting that it is a keystone mutualist (Culver and Beattie 1978; Zelikova et al. 2008; Ness et al. 2009). Thus, if P. chinensis displaces A. rudis and fails to disperse seeds, it has the potential to reduce seed dispersal in invaded areas, leaving seeds more susceptible to predation by rodents as well as competition with parent plants. Here, we test the hypothesis that P. chinensis disrupts ant-seed dispersal mutualisms in a forest ecosystem in the southeastern United States. Specifically, we ask whether the presence of P. chinensis is associated with (1) changes in the structure of the native ant community and reductions in the abundance of the keystone mutualist A. rudis, (2) reductions in seed removal, and (3) reductions in the abundances of myrmecochorous plants. (4) Finally, we compare the impacts of P. chinensis in our study sites with impacts of the invasive Argentine ant using tools from meta-analysis.
Materials and methods
Natural history
The native range of P. chinensis includes much of East Asia (Yashiro et al. 2010). Though the means and timing of its introduction into the US are unknown, this species likely invaded the southeastern US from Japan some time before 1932 (Smith 1934); where it remained relatively inconspicuous with small colonies of only a few hundred workers for several decades (McGown 2009). However, in the past 10 years P. chinensis has become widespread and is now locally abundant in parts of Alabama, Georgia, North Carolina, South Carolina, Tennessee and Virginia, with viable colonies as far north as New York state (Guénard and Dunn 2010).
We conducted this study from May to July of 2009 at the 232-ha Historic Yates Mill County Park, a mature closed-canopy, mesic deciduous forest in Raleigh, North Carolina, USA (35° 43′N and 78° 41′W). Temperature in the area ranges from a minimum of −1°C in January to a maximum of 32°C in July with mean annual precipitation of 1,052 mm year−1. Historic Yates Mill County Park is a conservation area and is dominated by Pinus taeda, Quercus spp., Carya spp. and Acer rubrum. It is adjacent to agricultural land. The understory consists of deciduous seedlings and numerous myrmecochorous plant species (Asarum canadense L., Hexastylis arifolia (Michx.), Trillium spp., Viola rotundifolia (Michx.), Sanguinaria canadensis L.) Our study focuses on two closely related myrmecochorous plant species in the family Aristolochiaceae (Birthwort), A. canadense and H. arifolia, which produce similar size and weight seeds (Canner 2010).
We haphazardly selected 29 invaded plots where P. chinensis was present and paired these with 29 intact plots (where P. chinensis was absent). Each plot was 10 m × 10 m. Invaded plots were in areas in which we observed P. chinensis foragers or active colonies. The intact plots were chosen by walking 20 m (twice the maximum distance of foraging by P. chinensis (Guénard personal observation) in a haphazardly chosen direction from the invaded plot. The intact plots were established where no P. chinensis foragers or nests were observed. Once the plot was selected, we extensively searched for P. chinensis workers and nests. This paired design has the advantage of partially controlling for environmental factors that might covary with the effects of P. chinensis.
Effect of P. chinensis on native ant assemblages
To sample the ants in each plot, we placed three pitfall traps, arranged in a triangle, with sides of 5 m. Pitfall traps were specimen cups 55 mm in diameter and 75 mm deep. These were partially filled with soapy water, buried flush with the ground, and left open for 24 h during non-rainy weather. Ants were sampled once at each plot. Pitfall traps are an effective method to sample the subset of ant species that are active ground foragers, which includes both P. chinensis and the native A. rudis (Bestelmeyer et al. 2000). The activity period of P. chinensis (time of the year where workers forage) runs from mid April to the end of October overlaping with the peak of activity of A. rudis in this system (B. Guénard, personal observation).
Effect of P. chinensis on seed dispersal
We assessed the impact of P. chinensis on seed dispersal rates of bloodroot (A. canadense), a common myrmecochorous species in Yates Mill County Park. The species is a widespread, shade-tolerant perennial that occurs in forest understories throughout the eastern US (Damman and Cain 1998). All seeds were collected close to the study site but not from the site itself. At each plot, we placed three seed depots of 10 seeds each at the corners of the triangles described above (prior to the placement of pitfall traps). We used 10 seeds in order to obtain results that are comparable to previous studies on seed removal by ants (Zettler et al. 2001; Rey et al. 2002; Ness 2004; Zelikova et al. 2008). Seed were collected in May of 2009 and kept frozen until the experiment began (see Zelikova et al. 2008). The use of preserved seeds did not lead to any bias in ant removal (Zelikova et al. 2008). Seeds were placed on wooden cards in the invaded plots and on laminated index cards in the intact plots. Wooden cards were used in the invaded plots because P. chinensis workers appeared to have some difficulty walking on the laminated index cards, a difficulty not shared by any of the other species. The wooden cards and index cards were similar in size and thickness. A pilot experiment demonstrated that card type (wooden vs. laminated paper) did not influence seed removal rates by native ant species. At each bait station, we recorded the identity of the ant species removing the seeds as well as the time taken to discover and remove the seeds from each of the cards for 1 h or until all of the seeds were removed, whichever came first. Seed presentations were conducted between the hours of 0900 and 1500 to coincide with a period of active foraging for most species in the study region (Fellers 1989).
Habitat characteristics
Within each plot we measured a suite of habitat variables representing the physical structure of the habitat within a 1-m radius circular plots centered on each card (n = 3 per plot) to determine what characteristics, other than the presence of P. chinensis, might differ between invaded and intact plots. These included number of logs (>12 cm in diameter), number of branches (2.5–12 cm in diameter) and number of sticks (<2.5 cm in diameter). Additionally, we measured the ground surface temperature of the leaf litter just outside of the four corners of each card using a handheld infrared thermometer (Raytek® Raynger ST) to estimate the surface temperature of the ground in each plot.
Comparison of the effect of P. chinensis with the invasive Argentine ant
We calculated the effect of P. chinensis on the number of seed dispersers (A. rudis workers) and on the number of seeds removed (effect size) as the log-response ratio (ln R),
where \( \overline{X}^{P} \) is the mean of the response variable in the invaded plots and \( \overline{X}^{A} \) is the mean of the response in the intact plots (following Rodriguez-Cabal et al. 2009). We performed a one-sample t-test to compare the magnitude of the impact of P. chinensis with the impact of the Argentine ant as determined in a previous study (Rodriguez-Cabal et al. 2009).
Data analysis
We performed paired t-tests to compare several characteristics of invaded and intact plots: total number of seeds removed; number of seeds removed by each ant species; time to removal of the first seed; number of myrmecochorous plants; number of logs, branches and sticks and ground temperatures. We log transformed time to removal of the first seed to improve normality and reduce heteroscedasticity. Additionally, we performed linear regression analyses to evaluate the relationship between the abundance of P. chinensis and A. rudis on the number of seed removed. Finally, we performed separate linear regression analyses to examine the relationship between abundances of P. chinensis, A. rudis and myrmecochorous plants against the habitat variables representing the physical structure of the habitat in invaded and intact plots.
Results and discussion
Plants that depend on ants as seed dispersers often rely disproportionately on a single ant species or species group (Gove et al. 2007; Manzaneda and Rey 2009; Ness et al. 2009), making them especially vulnerable if the behaviour or abundance of the keystone mutualist is altered (Giladi 2006). Many plant species in the eastern US rely on A. rudis for seed dispersal (Ness et al. 2009). If the abundance of this ant species group is decreased by the invasion of P. chinensis, seed dispersal hence has the potential to be altered. In addition to the invasive ant P. chinensis we also found A. rudis, A. pallipes, F. subsericea, C. pennsylvanicus, C. castaneus and C. americanus within invaded plots. In the intact plots we found A. rudis, F. subsericea, C. pennsylvanicus, C. castaneus, C. americanus, T. curvispinosus. However, species density was greater in invaded plots (mean ± SE, 2.13 ± 1.06) than intact plots (1.43 ± 1.04) (t = 2.25, n = 23, P < 0.05). We found that the number of A. rudis workers was 96% lower in invaded than in intact plots (t = −4.58, n = 23, P < 0.0001; Fig. 1). Additionally, we found A. pallipes only in invaded plots (t = 2.75, n = 23, P < 0.05) and only three workers of T. curvispinosus in intact plots. Conversely, the presence of P. chinensis was not associated with the number of individuals of F. subsericea (t = 0.65, n = 23, P = 0.52), C. pennsylvanicus (t = 0.78, n = 23, P = 0.44), C. castaneus (t = 1.04, n = 23, P = 0.31) or C. americanus (we found only two workers of C. americanus, one at an intact plot and one at one invaded plot). These results are similar to results from Guénard and Dunn (2010), who reported that species of both Formica and Camponotus were either more common or unaffected by the presence of P. chinensis. Studies on other, more well-studied, invasive species have found that not all native ant species are equally affected by the spread of an invasive species (Porter and Savignano 1990; Suarez et al. 1998; Holway et al. 2002; Lessard et al. 2009). One possible explanation for the dramatic drop in A. rudis abundance could be that P. chinensis is preferentially preying upon A. rudis. Pachycondyla chinensis has been found to kill A. rudis workers in direct interactions in laboratory experiments (Bednar 2010). Another possible explanation for this pattern may be that P. chinensis and A. rudis compete for nest sites. As is the case with many ant invasions, distinguishing between the effects of interspecific competition and predation is often challenging (Holway et al. 2002).
Comparison of the number of ants (mean ± 1 SE) in plots invaded by P. chinensis invaded and intact plots. Plots with pitfall traps lacking P. chinensis were recorded as intact sites (n = 29). Significant differences in number of ants between invaded and intact sites are noted with an asterisk (P. chinensis t = 4.52, P < 0.0002; A. rudis t = −4.58, P < 0.0001; F. subsericea t = 0.64, P = 0.52; C. pennsylvanicus t = 0.78, P = 0.44; C. castaneus t = 1.03, P = 0.31)
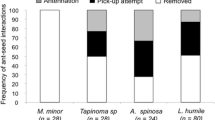
The efficiency of a seed disperser is the contribution of a disperser to the fitness of a plant, and this measure involves both the quantity and quality of dispersal (Schupp 1993). The quantitative component is the number of seeds dispersed and the quality depends on the probability that a dispersed seed will become an adult. We found that the number of seeds removed per plot was 70% lower in invaded than in intact plots (t = −10.69, n = 29, P < 0.0001). In invaded plots, the majority of seeds were removed by P. chinensis, which accounted for 97% of the 243 seeds removed, whereas A. rudis was responsible for 96% of the 773 seeds removed in the intact plots (Fig. 2). Moreover, we found a negative relationship between the number of seeds removed and the number of P. chinensis workers present in plots (R 2 = 0.22, P = 0.001); in contrast, we found a positive relationship with the number of A. rudis workers and the number of seeds removed (R 2 = 0.18, P = 0.003). Consequently, P. chinensis affects seed removal and potentially dispersal by removing fewer seeds than does A. rudis in intact areas. A growing body of work shows that invasive ants seem to be inferior seed dispersers to native ants, often finding seeds more slowly (Gómez and Oliveras 2003). In our system, because of the numerical dominance of P. chinensis in invaded plots and their relatively low rates of seed dispersal, seeds in invaded plots were discovered two times more slowly than they were in intact plots (16.87 ± 1.67 min vs. 8.36 ± 1.31 min) (t = 4.01, P < 0.0003).
Mean (±1 SE) number of A. canadense seeds removed by ants species in 29 invaded and intact plots. Significant differences in number of seeds removed by each ant species between invaded and intact sites are noted with an asterisk (P. chinensis t = 5.24, P < 0.0001; A. rudis t = −28.89, P < 0.0001; F. subsericea t = −1.27, P = 0.21; C. pennsylvanicus t = −1.21, P = 0.23; others (other ants and insects) t = −1.80, P = 0.08)
As a consequence of the low quantity of seed removed by the invasive P. chinensis, many seeds in invaded plots may simply not be dispersed. As for those seeds that are removed by P. chinensis, their fate is unknown. Eguchi (2004) suggested that P. chinensis in its native rage might act as a granivorous species. Another study considered P. chinensis a poor seed disperser despite being native to a region where elaiosome-bearing seeds are common (Yashiro et al. 2010). This invasive ant has been described as a termite specialist but also consumes other animals in its invaded range (Guénard and Dunn 2010). Seeds dispersed by A. rudis are generally left in middens inside nests (which are either in logs or the litter) or carried out of nests into the leaf-litter (Culver and Beattie 1980). We do not know if seed removed by P. chinensis are being predated or dispersed. However, because P. chinensis nests tend to be inside logs, we presume that most of the seeds collected by P. chinensis end up buried in or beneath logs, which likely affects the quality of the seed dispersal. We do not know whether seeds deposited under logs have higher (as would be the case if logs serve as ‘nurse logs’) or lower germination success than those on the soil surface or on the trash pile.
Hexastylis arifolia was the only myrmecochorus plant species conspicuous at the time our study was conducted because the leaves and flowers of A. canadense die after the growing season, leaving only the rhizome to overwinter (Cain and Damman 1997). The density of H. arifolia was 50% lower in invaded plots (1.43 ± 0.30) than in intact plots (3.14 ± 0.65) (t = −2.42, n = 29, P < 0.05). This result is reconcilable with the effects of reduced levels of seed dispersal caused by the dramatic reduction of A. rudis (Zelikova et al. 2008; Ness et al. 2009). Moreover, H. arifolia seeds do not persist in the seed bank (Giladi 2004). Consequently, A. rudis is clearly an important seed disperser—the richness of myrmecochorous plants in temperate hardwood forests of eastern North America is often positively related to the abundance of A. rudis (Mitchell et al. 2002), and Zelikova et al. (2008) found that A. rudis was responsible for ~99% of seed dispersal events in Great Smoky Mountains in the southeastern US. Other studies of the effects of invasive ants on seed dispersal mutualisms have found similar results. For example, the effect of L. humile on the plant community composition of the Fynbos in South Africa arises because of the disruption of seed dispersal mutualisms (Bond and Slingsby 1984; Christian 2001). However, plants in the Fynbos do not rely on a single species of ant (Christian 2001). In our system (and perhaps more generally in forests of the eastern US), a single ant species complex (A. rudis is probably a complex of 3–4 species) is responsible for the majority of seed dispersal events. Although P. chinensis does not appear to cause declines in the abundance of all ant species (Guénard and Dunn 2010), it appears to affect the seed disperser and in doing so affects the process, seed-dispersal, that species mediates.
The differences in the densities of H. arifolia between invaded and intact plots could have resulted from factors other than changes in seed dispersal. For example, it may have arisen because of differences in rates of herbivory in intact and invaded plots, if P. chinensis does not prey on the herbivores of H. arifolia to the same extent as native ant species do (Holway et al. 2002; Styrsky and Eubanks 2010). However, the percentage of H. arifolia with apparent damage by herbivores did not differ between invaded (12% ± 3.60) and intact plots (7% ± 2.60) (t = 1.08, n = 23, P = 0.29). Additionally, we estimated possible rates of predation on invertebrates in intact and invaded plots by placing four wax-worms (previously frozen) at the center of each plot and recorded the number of worms removed after 24 h. We found no difference in the number of wax-worms removed in the invaded (3.9 ± 0.1) and intact (4.0 ± 0) plots (t = −1.00, n = 10, P = 0.34). Together, these results suggest that the indirect positive effects of deterring herbivores insects by P. chinensis on H. arifolia, if there are any, do not differ from the effects of native ant species. It seems likely that the lower density of H. arifolia in invaded plots relative to intact plots is not likely due to differential herbivory.
An alternative explanation for the differences in H. arifolia abundance between intact and invaded plots is that the plots differed in their abiotic or biotic conditions. Abiotic and biotic variables might influence the establishment of myrmecochorous plants in this system, and that these same abiotic and biotic variables also influence the abundances of A. rudis and P. chinensis. We found more logs in plots invaded by P. chinensis than in intact plots (invaded = 0.88 ± 0.11 vs. intact = 0.43 ± 0.10; t = 3.14, n = 29, P < 0.05), but we found no differences in the number of branches (t = −1.24, n = 29, P = 0.22) and sticks between invaded and intact plots (t = −0.65, P = 0.51). From a pitfall survey conducted at each plot we did not find a relationship between the number of logs and P. chinensis abundance (R 2 = 0.05, P = 0.27), nor was there a relationship between the abundance of A. rudis and the number of logs (R 2 = 0.01, P = 0.62). Likewise, there was no relationship between the number of logs and the density of myrmecochorous plants in invaded s (R 2 = 0.07, P = 0.12) or in intact plots (R 2 = 0.08, P = 0.16). Additionally, we did not find differences in ground temperature between invaded and intact plots (invaded = 24.80 ± 3.14°C vs. intact = 24.41 ± 1.79°C; t = −0.59, n = 29, P = 0.56). These results suggest a causal relationship between the decreased abundance of A. rudis and the number of myrmecochorous plants in invaded sites. We did not test for any other abiotic variables, though, because of the proximity of the invaded and intact sites, we suppose that the strength of the abiotic variables is similar among these sites.
We used meta-analysis to compare the effects observed in this study to the effects of Argentine ants, L. humile, on seed dispersal mutualisms. We found the effects of P. chinensis and L. humile to be quantitatively similar: the presence of the invasive Argentine ant led to a 92% reduction in the abundance of native ant seed dispersers (Rodriguez-Cabal et al. 2009), and the presence of P. chinensis led to a the 96% reduction (t = −0.34, n = 7, P = 0.74). Additionally, the effect of P. chinensis on the number of seeds dispersed did not differ from the effects of Argentine ants (t = 0.81, n = 18, P = 0.43): plots invaded by P. chinensis had, on average, 70% fewer seeds dispersed, similar to the 76% reduction in the number of seeds dispersed in plots with Argentine ants (Rodriguez-Cabal et al. 2009). While there is some question as to whether invasive ants such as S. invicta and L. humile become established in plots where native ant diversity is already reduced or whether they reduce diversity (Sanders and Suarez 2010; Stuble et al. 2011), several lines of evidence strongly suggest that P. chinensis is responsible for the declines in the abundance of the keystone seed-dispersers ant in this system. First, our study plots occur in protected forests that have been minimally impacted by human disturbance over at least the past several decades. Other invasive ants (S. invicta, L. humile), which are sometimes associated with disturbance, are common near our study plots but have not been detected to date in our study plots. Second, our matched pair design should to some extent account for the variation in habitat characteristics, other than the presence of P. chinensis.
In conclusion, our results indicate that P. chinensis is associated with the disruption of an ant-plant seed dispersal mutualism and is potentially reducing abundance of ant-dispersed plants. Our study is in line with previous studies that have documented the negative effects of invasive ants on ant-plant seed dispersal mutualisms (Bond and Slingsby 1984; Christian 2001; Carney et al. 2003; Ness and Bronstein 2004; Rodriguez-Cabal et al. 2009) and documents this detrimental effect of an understudied invasive ant species on an important ecological interaction. Finally, our study focused on two myrmecochorous species. Examining the community-level impacts of invasive species, or the impacts on the suite of myrmecochorous species, would be an exciting and largely untapped area for future research.
References
Bascompte J, Jordano P, Melian CJ, Olesen JM (2003) The nested assembly of plant-animal mutualistic networks. Proc Nat Acad Sci USA 100:9383–9387
Bascompte J, Jordano P, Olesen JM (2006) Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 312:431–433
Bednar DM (2010) Pachycondyla (=Brachyponera) chinensis predation on Reticuletermes virginicus and competition with Aphaenogaster rudis. Master of science thesis, North Carolina State University, USA
Bestelmeyer BT, Agosti D, Alonso LE, Brandão CRF, Brown WL Jr, Delabie JHC, Silvestre R (2000) Field techniques for the study of ground-dwelling ants: an overview, description, and evaluation. In: Agosti D, Majer JD, Alonso LE, Schultz TR (eds) Ants: standard methods for measuring and monitoring biodiversity. Smithsonian Institution Press, Washigton, pp 122–144
Bond W, Slingsby P (1984) Collapse of an ant-plant mutualism: the Argentine ant (Iridomiyrmex humilis) and myrmecochorous Proteaceae. Ecology 65:1031–1037
Cain ML, Damman H (1997) Clonal growth and ramet performance in the woodland herb, Asarum canadense. J Ecol 85:883–897
Canner JE (2010) The population ecology of ant-dispersed plants in space and time. Dissertation, North Carolina State University, USA
Carney SE, Byerley MB, Holway DA (2003) Invasive Argentine ants (Linepithema humile) do not replace native ants as seed dispersers of Dendromecon rigida (Papaveraceae) in California, USA. Oecologia 135:576–582
Christian CE (2001) Consequences of a biological invasion reveal the importance of mutualism for plant communities. Nature 412:635–639
Culver DC, Beattie AJ (1978) Myrmecochory in Viola: dynamics of seed-ant interactions in some West Virginia species. J Ecol 66:53–72
Culver DC, Beattie AJ (1980) The fate of Viola seeds dispersed by ants. J Bot 67:710–714
Damman H, Cain ML (1998) Population growth and viability analyses of the clonal woodland herb, Asarum canadense. J Ecol 86:13–26
D’Antonio CM, Vitousek PM (1992) Biological invasions by exotic grasses, the grass fire cycle, and global change. Annu Rev Ecol Syst 23:63–87
Eguchi K (2004) A survey on seed predation by omnivorous ants in the warm-temperate zone of Japan (Insecta, Hymnoptera, Formicidae). New Entomol 53:7–18
Ellison AM, Bank MS, Clinton BD, Colburn EA, Elliott K, Ford CR, Foster DR, Kloeppel BD, Knoepp JD, Lovett GM, Mohan J, Orwig DA, Rodenhouse NL, Sobczak WV, Stinson KA, Stone JK, Swan CM, Thompson J, von Holle B, Webster JR (2005) Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Front Ecol Environ 9:479–486
Fellers JH (1989) Daily and seasonal activity in woodland ants. Oecologia 78:69–76
Giladi I (2004) The role of habitat-specific demography, habitat specific dispersal, and the evolution of dispersal distances in determining current and future distributions of the ant dispersed forest herb, Hexastylis arifolia. Dissertation, University of Georgia, USA
Giladi I (2006) Choosing benefits or partners: a review of the evidence for the evolution of myrmecochory. Oikos 112:481–492
Gilbert LE (1980) Food web organization and the conservation of neotropical diversity. In: Soule ME, Wilcox BA (eds) Conservation biology: an evolutionary-ecological perspective. Sinauer, Sunderland, pp 11–33
Gómez C, Oliveras J (2003) Can the Argentine ant (Linepithema humile Mayr) replace native ants in myrmecochory? Acta Oecol 24:47–53
Gómez C, Pons P, Bas JM (2003) Effects of the Argentine ant Linepithema humile on seed dispersal and seedling emergence of Rhamunus alaternus. Ecography 26:532–538
Gove AD, Majer JD, Dunn RR (2007) A keystone ant species promotes seed dispersal in a “diffuse” mutualism. Oecologia 153:687–697
Guénard B, Dunn RR (2010) A new (old), invasive ant in the hardwood forests of Eastern North America and its potentially widespread impacts. PLoS ONE 5(7):e11614. doi:10.1371/journal.pone.0011614
Holway DA, Lach L, Suarez AV, Tsutsui ND, Case TJ (2002) The causes and consequences of ant invasions. Annu Rev Ecol Syst 33:181–233
Howe HF, Smallwood J (1982) Ecology of seed dispersal. Annu Rev Ecol Syst 13:201–228
Jordano P, Schupp EW (2000) Determinants of seed disperser effectiveness: the quantity component and patterns of seed rain for Prunus mahaleb. Ecol Monogr 70:591–615
Lessard JP, Fordyce JM, Gotelli NJ, Sanders NJ (2009) Invasive ants alter the phylogenetic structure of native communities. Ecology 90:2664–2669
Levine JM, Vilà M, D’Antonio CM, Dukes JS, Grigulis K, Lavorel S (2003) Mechanism underlying the impacts of exotic plant invasions. Proc Royal Soc B 270:775–781
Manzaneda AJ, Rey PJ (2009) Assessing ecological specialization of an ant-seed dispersal mutualism through a wide geographic range. Ecology 90:3009–3022
McCoy NL (2009) The geographical mosaic of myrmecochory in a global biodiversity hotspot and the fate of myrmecochorous seeds dispersed by a keystone seed disperser. Master of science thesis, North Carolina State University, USA
McGlynn T (1999) The worldwide transfer of ants: geographical distribution and ecological invasions. J Biogeogr 26:535–548
McGown JA (2009) The Asian Needle Ant, Pachycondyla chinensis (Emery)(Hymenoptera: Formicidae), reported from Alabama. Midsouth Entomol 2:88–89
Mitchell CE, Turner MG, Pearson SM (2002) Effects of historical land use and forest patch size on myrmecochores and ant communities. Ecol Appl 12:1364–1377
Ness JH (2004) Forest edges and fire ants alter the seed shadow of an ant-dispersered plant. Oecologia 138:228–454
Ness JH, Bronstein JL (2004) The effects of invasive ants on propective ant mutualists. Biol Invasions 6:445–461
Ness JH, Morin DF, Giladi I (2009) Uncommon specialization in a mutualism between a temperate herbaceous plant guild and an ant: are Aphaenogaster ants keystone mutualists? Oikos 118:1793–1804
Porter SD, Savignano DA (1990) Invasion of polygyne fire ants decimates native ants disrupts arthropod community. Ecology 71:2095–2106
Rey PJ, Garrido JL, Alcántara JM, Ramírez JM, Aguilera A, García L, Manzaneda AJ, Fernández R (2002) Spatial variation in ant and rodent post-dispersal predation of vertebrate-dispersed seeds. Funct Ecol 16:773–781
Rodriguez-Cabal MA, Aizen MA, Novaro AJ (2007) Habitat fragmentation disrupts a plant-disperser mutualism in the temperate forest of South America. Biol Conserv 139:195–202
Rodriguez-Cabal MA, Stuble KL, Nuñez MA, Sanders NJ (2009) Quantitative analysis of the effects of the exotic Argentine ant on seed-dispersal mutualisms. Biol Lett 5:499–502
Rowles AD, O’Dowd DJ (2009) New mutualism for old: indirect disruption and direct facilitation of seed dispersal following Argentine ant invasion. Oecologia 158:709–716
Sanders NJ, Suarez AV (2010) Elton’s insights into the ecology of ant invasions: lessons learned and lessons still to be learned. In: Richardson DM (ed) Fifty years of invasion ecology: the legacy of Charles Elton. Blackwell, New York, pp 239–251
Schupp EW (1993) Quantity, quality and the effectiveness of seed dispersal by animals. Vetatio 107(108):15–29
Schupp EW, Fuentes M (1995) Spatial patterns of seed dispersal and the unification of plant population ecology. Ecoscience 2:267–275
Smith MR (1934) Ponerine ants of the genus Euponera in the United States. Ann Entomol Soc Am 27:558–564
Stuble KL, Kirkman LK, Carroll CR (2010) Are red imported fire ants facilitators of native seed dispersal? Biol Invasions 12:1661–1669
Stuble KL, Kirkman LK, Carrol CR, Sanders NJ (2011) Relative effects of disturbance on red imported fire ants and native ant species in a longleaf pine ecosystem. Conserv Biol 25:618–622
Styrsky JD, Eubanks MD (2010) A facultative mutualism between aphids and an invasive ant increases plant reproduction. Ecol Entomol 35:190–199
Suarez AV, Bolger DT, Case TJ (1998) Effects of fragmentation and invasion on native ant communities in coastal southern California. Ecology 79:2041–2056
Tardiff SE, Stanford JA (1998) Grizzly bear digging: effects on subalpine meadow plants in relation to mineral nitrogen availability. Ecology 79:2219–2228
Traveset A, Richardson DM (2006) Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol Evol 21:208–216
Vazquez DP, Aizen MA (2004) Degree distribution in plant-animal mutualistic networks: forbidden links or random interactions. Oikos 108:421–426
Vazquez DP, Simberloff D (2004) Indirect effects of an introduced ungulate on pollination and plant reproduction. Ecol Monogr 74:281–308
Vazquez DP, Morris WF, Jordano P (2005) Interaction frequency as surrogate for the total effect of animal mutualists on plants. Ecol Lett 8:1088–1094
Vitousek PM (1990) Biological invasions and ecosystem processes—towards an integration of population biology and ecosystem studies. Oikos 57:7–13
Wenny DG, Levey DJ (1998) Directed seed dispersal by bellbirds in a tropical cloud forest. Proc Natl Acad Sci USA 95:6204–6207
Yashiro T, Matsuura K, Guénard B, Terayama M, Dunn RR (2010) On the evolution of the species complex Pachycondyla chinensis (Hymenoptera: Formicidae: Ponerinae), including the origin of its invasive form and description of a new species. Zootaxa 2685:39–50
Zelikova TJ, Dunn RR, Sanders NJ (2008) Variation in seed dispersal along an elevation gradient in Great Smoky Mountains National Park. Acta Oecol 34:155–162
Zettler JA, Spira TP, Allen CR (2001) Ant–seed mutualisms: can red imported fire ants sour the relationship? Biol Conserv 101:249–253
Acknowledgments
We thank Historic Yates Mill County Park for permission to carry out field work and G. L. McCormick and J. E. Canner for assistance in the field. M. N. Barrios-Garcia, J. A. Schweitzer, J. K. Bailey and M. A. Nuñez provided advice that greatly improved this manuscript. This research was funded by the Department of Ecology and Evolutionary Biology at the University of Tennessee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodriguez-Cabal, M.A., Stuble, K.L., Guénard, B. et al. Disruption of ant-seed dispersal mutualisms by the invasive Asian needle ant (Pachycondyla chinensis). Biol Invasions 14, 557–565 (2012). https://doi.org/10.1007/s10530-011-0097-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-011-0097-5