Abstract
Here we present the first observation of the impact of the invasive Caulerpa racemosa var. cylindracea on native photophilic sponge species in the Adriatic Sea, with special focus on Sarcotragus spinosulus. Caulerpa racemosa var. cylindracea is able to completely overgrow the sponge, developing an exceptionally thick canopy with a maximum measured density of 1,887 m of stolons m−2 and 40,561 fronds m−2. Necrosis of the sponge surface was significantly correlated with the algal dry biomass, frond number and stolon length. Dense algal canopy, penetration of the algal stolon and rhizoids into the sponge oscula and covering of the ostiae probably diminishes the seawater circulation through the sponge and consequently results in its smothering and even death. We suggest that chemotropism is the reason why C. racemosa penetrates the sponge oscula and establishes such dense canopy on the sponge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The invasive alga Caulerpa racemosa (Forskål) C. Agardh var. cylindracea Verlaque, Huisman et Boudouresque (C. racemosa from here after) was observed in the Mediterranean Sea for the first time in the early 1990s. Since then it has been reported from 14 Mediterranean countries and on the Canary Islands in the Atlantic (Verlaque et al. 2000, 2003; Piazzi et al. 2005; Klein 2007; Ould-Ahmed and Meinesz 2007; Cottalorda et al. 2008; Klein and Verlaque 2008).
Caulerpa racemosa has siphonous thallus divided into a branched creeping axis (stolon), which is 0.7–2.0 mm in diameter and erect shoots (fronds) usually up to 11 cm long. The thallus is fixed to the substratum by thin rhizoids, from 1 to 20 mm long that are closely arranged along the stolon (Verlaque et al. 2003). In the Mediterranean it is a perennial species and can form an exceptionally dense canopy with maximum biomass in the autumn. A density of over 2,600 m of stolons m−2 and 27,000 fronds m−2 has been measured in the middle Adriatic (Žuljević et al. 2003) and over 1,500 m of stolons m−2 and 32,000 fronds m−2 in the north Adriatic (Iveša and Devescovi 2006). It can grow on all substrate types and at depths up to 70 m (Piazzi et al. 2005; Ruitton et al. 2005).
To date, there have only been a few studies on the impact of C. racemosa on Mediterranean fauna. In Sardinia (Italy), no influence on the rocky infralittoral zoobenthic assemblages was detected (Casu et al. 2005). Piazzi and Balata (2008) found that on rocky substrates sessile fauna were less abundant in invaded than in non-invaded assemblages and bryozoans were completely absent. On shallow rocky substrates in the south-eastern Spain, species diversity of amphipods was high and not affected by C. racemosa, while the species composition changed completely in the invaded area (Vázquez-Luis et al. 2008). Inside dense C. racemosa canopy in the middle Adriatic Sea, the number of species and abundance of epiphytic algae on Posidonia oceanica (L.) Delile rhizomes is drastically reduced (Antolić et al. 2008). In the Ionian Sea it was found that C. racemosa in coralligenous concretions diminishes the percentage of sponge cover during 2 years of invasion (Baldacconi and Corriero 2009).
Since the first observation of C. racemosa in Croatia in 2000 (Žuljević et al. 2003), we have noted that the alga has the ability to overgrow some photophilic sponge species with exceptional cover density. Here we have documented the impact of C. racemosa on different photophilic sponge species with particular focus on Sarcotragus spinosulus Schmidt 1862.
Materials and methods
Field observations were made at several locations invaded by C. racemosa, in different periods of the year, along the Croatian coast to determine if the impact of C. racemosa on photophilic sponges is a widespread phenomenon.
Sponge specimens overgrown by C. racemosa were collected in Priježba Bay (Pelješac peninsula, Croatia) (42° 47.605′N, 17° 44.125′E) on the 11th October 2002 (Fig. 1) at depths between 3 and 10 m.
Among the collected sponges, we analyzed 12 specimens of photophilic sponges Sarcotragus spinolusus, which were completely overgrown by Caulerpa racemosa (Fig. 2e). Within 1 h after collecting, the algae were removed from the sponge and percentage of surface necrosis on the sponge was visually estimated. On the fresh algae, total stolon length was measured and number of fronds was counted. Dry algal mass was weighed separately for stolons and fronds after 48 h drying at 80°C.
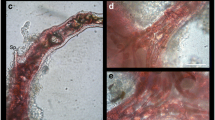
Different aspects of Sarcotragus spinosulus colonized by Caulerpa racemosa. Early stage (a, b) and advanced stage (c, d) of colonisation. Fully overgrown sponge (e) and S. spinosulus after removal of C. racemosa (f). In total 31 m of stolons and 860 fronds (e) have been developed on the sponge of 10 cm in diameter (f). The sponge was completely necrotized and it had characteristic strong hydrogen sulphide odour. This sample was not included in the analysis
Sponge surface area and diameter was calculated from the volume data, assuming the sponge to be spherical which mostly corresponds to the shape of Sarcotragus spinosulus (Fig. 2a). Volume was measured using water displacement of the frozen wet sponge. For each sponge specimen, species identification was rechecked in the laboratory. Dry algal biomass and stolon and frond density per surface area of the sponge were calculated, assuming homogeneous distribution of the algae on the sponge surface.
Correlations between stolon length and frond density per surface of the sponge and the percentage of necrosis were tested with Pearson’s correlation.
Results
Photophilic sponge species overgrown by Caulerpa racemosa have been observed at almost every C. racemosa location in the Croatian part of the Adriatic Sea (for a map of locations see http://jadran.izor.hr/kaulerpa/). It is a widespread phenomenon, which can be observed throughout the year. On the well-lit rocky bottom affected by C. racemosa between 2 and 10 m deep, it is common to find sponges partially or completely covered by the alga (Fig. 2; Online Resource – Video 1). A completely covered sponge looks like a ball of C. racemosa (Fig. 2c, d, e; Online Resource – Video 1). Such balls consisting of decomposing sponge body in the center of dense C. racemosa canopy were also found at some locations. Density of C. racemosa in the area around the fully overgrown sponge is always lower compared to multilayer algal canopy on the sponge. A white mat of the anoxic bacteria Beggiatoa spp. was frequently visible under the stolons on sponges completely covered with C. racemosa. Such samples always had a characteristic hydrogen sulphide odour.
Sarcotragus spinolusus, Aplysina aerophoba, Petrosia ficiformis and Ircinia sp. were the species affected and densely overgrown by C. racemosa that were possible to recognize in situ (in situ identification is difficult or impossible for the majority of the sponge species).
The completely overgrown specimens of Sarcotragus spinosulus collected were of small size (diameter between 4.7 cm and 10.2 cm, n = 12) (Table 1). Even on the sponge that small, C. racemosa developed high density of stolons and fronds (Fig. 1.). Stolon density ranged from 305 to 1,887 m of stolons m−2 while the frond density was between 10,366 and 40,561 fronds m−2 (Table 1).
Necrosis of the sponge surface is strongly correlated with the frond density (r = 0.73), stolon density (r = 0.76) and dry algal biomass (r = 0.76).
According to measured mean dry biomass of C. racemosa (stolons + fronds) on the sponges (expressed as algal biomass per sponge surface), stolons represent a slightly higher fraction (56.4%) than fronds (43.6%) (Table 1). Dry frond biomass is highly correlated with dry stolon biomass (r = 0.74), as well as density of fronds with density of stolon (r = 0.91).
Discussion
Caulerpa species use at least some sponge species as a favorable growing substrate which consequently might lead to sponge mortality. An impact to the sponge assemblage from coralligenous communities was reported from the Ionian Sea. During a 2-year study Baldacconi and Corriero (2009) found that the spread of C. racemosa was associated with a slight decrease in the number of sponge species and with significant decrease in percent cover of sponges. A rapid invasion by Caulerpa scalpelliformis on a sponge dominated deep-reef and overgrowing of incrusting sponges by the algal stolons, has been reported in Australia (Davis et al. 1997). We recorded that Caulerpa taxifolia, the second invasive Caulerpa in the Mediterranean, is also able to grow on sponges (pers. obs.) although never reaching the same high density as C. racemosa presented here.
Density of C. racemosa canopy on fully overgrown sponge Sarcotragus spinolusus is exceptional and even higher than algal density measured on any other substratum up to date. The extreme algal density in the Mediterranean Sea was measured in Vrsar (North Adriatic) with around 32,000 fronds m−2 and 1,200 m of stolons m−2 (Iveša and Devescovi 2006), and in Pakleni island (Middle Adriatic) with 27,000 fronds m−2 and 2,600 m of stolons m−2 (Žuljević et al. 2003), both colonies were established on the rocky bottom. Here we are reporting the very high density of 40,561 fronds m−2 and 1,887 m of stolons m−2 which the algae developed on the sponge Sarcotragus spinolusus (Table 1).
Such density is even more intriguing because we know that Sarcotragus spinosulus is usually free of epibionts and epiphytes, due to a large number of secondary metabolites (Pawlik et al. 2002; Tsoukatou et al. 2002; Choi et al. 2004; Wang and Shin 2008). Some other sponges like Crambe crambe which is resistant to epiphytes (pers. obs.) is resistant to overgrowth by C. racemosa, probably due to anti-fouling toxin (Baldacconi and Corriero 2009).
We think that the beginning of the algal invasion is stimulated by sponge excretions. In the early invasion stages we could clearly see that stolons and rhizoids penetrate the sponge through the oscula where the sponge releases the excretions (Fig. 2b). Caulerpa species have symbiotic bacteria inside their rhizoids allowing a nutrient uptake (Chisholm et al. 1996). This ability probably allows the algae to use the excretion products of the sponge. Elongation of the algal stolon into the osculum, the area of high concentration of sponge excretions, suggests a positive chemotropism. Exceptionally high algal density developed only on the sponge but not on the nearby rocky substrate (Fig. 2b), also suggests a chemotropic behavior.
Aside from chemotaxis in sexual cells of some brown and green algae (Maier and Müller 1986), to the best of our knowledge, there are no data on movements or growing of the macroalgal thalli induced by chemical stimuli.
Therefore, further research should be done to test the hypothesis of a chemotropic relationship between Caulerpa and sponges.
In the later stages of the invasion, C. racemosa completely overgrows the sponge and blocks the oscula and ostiae of the sponge. The C. racemosa becomes a physical barrier and diminishes the seawater circulation through the sponge thereby smothering it. This does not occur on the whole sponge at the same time, which is evident from only partial necrosis of the sponge surface. When parts of the sponge die, bio-decomposition probably further stimulates algal proliferation because it serves as an influx of nutrients for the algae.
Bio-decomposition that uses oxygen and dense algal canopy which reduces fresh water supply, results in anoxia and eventually leads to death of the entire sponge. Anoxic conditions are indicated with the presence of mat of anoxic bacteria Beggiatoa spp. and characteristic hydrogen sulphide odour of the fully overgrown sponge.
Time required for sponges to be completely overgrown by C. racemosa has not yet been determined, but it is likely happening during one growing season, considering stolon elongation of C. racemosa is 1.26 cm a day (Piazzi and Cinelli 1999) and that alga is able to cover 100% of a 400 cm2 quadrat forming few layers of interlaced stolons in just 6 months (Piazzi et al. 2001).
Apart from impact on sponge biodiversity, this exceptional growth of C. racemosa on some sponge species in the Mediterranean might have also a socio-economic impact if the algae affects the harvesting sponge in the exploiting areas in Croatia, Greece, Tunisia and Turkey (Vacelet 1991).
References
Antolić B, Žuljević A, Despalatović M, Grubelić I, Cvitković I (2008) Impact of the invasive green alga Caulerpa racemosa var. cylindracea on the epiphytic macroalgal assemblage of Posidonia oceanica seagrass rhizomes in the Adriatic Sea. Nova hedwigia 86(1–2):155–167
Baldacconi R, Corriero G (2009) Effects of the spread of the alga Caulerpa racemosa var. cylindracea on the sponge assemblage from coralligenous concretions of the Apulian coast (Ionian Sea, Italy). Mar Ecol 30:337–345
Casu D, Ceccherelli G, Palomba D, Curini-Gelletti M, Castelli A (2005) Effetto immediato della rimozione di Caulerpa racemosa sullo zoobenthos dell’infralittorale superficiale roccioso di Porto Torres (Nord Sardegna). In: XV Meeting of the Italian Society of Ecology, pp 1–3
Chisholm JRM, Dauga C, Ageron E, Grimont PAD, Jaubert JM (1996) “Roots” in mixotrophic algae. Nature 381:382
Choi K, Hong J, Lee C, Kim D, Sim C, Im K, Jung J (2004) Cyotoxic furanoses-tertepenes from a marine sponge Psammocinia sp. L Nat Prod 67:1186–1189
Cottalorda JM, Gratiot J, Mannoni PA, De Vaugelas J, Meinesz A (2008) Suivi de l’invasion des algues introduites Caulerpa taxifolia et Caulerpa racemosa en Méditerranée: situation devant les côtes françaises au 31 décembre 2007. E.A. 4228 ECOMERS, Laboratoire Environnement Marin Littoral - Université de Nice-Sophia Antipolis publ.: 42 pp+96 pages d’annexes
Davis AR, Roberts DE, Cummins SP (1997) Rapid invasion of a sponge dominated deep-reef by Caulerpa scalpeliformis (Chlorophyta) in Botany Bay, New South Wales. Austral J Ecol 22:146–150
Iveša Lj, Devescovi M (2006) Seasonal vegetation patterns of the introduced Caulerpa racemosa (Caulerpales, Chlorophyta) in the northern Adriatic Sea (Vrsar, Croatia). Period Biol 108(2):111–116
Klein J (2007) Impact de Caulerpa racemosa var. cylindrea (Caulerpales, Chlorophyta) sur les communautés macrophytiques en Méditerranée nord-occidentale. Thèse de Doctorat, Université Aix-Marseille II, France
Klein J, Verlaque M (2008) The Caulerpa racemosa invasion: a critical review. Mar Pollut Bull 56:205–225
Maier I, Müller DG (1986) Sexual pheromones in algae. Biol Bull 170:145–175
Ould-Ahmed N, Meinesz A (2007) First record of the invasive alga Caulerpa racemosa (Caulerpales, Chlorophyta) on the coast of Algeria. Cryptogam Algol 28:303–305
Pawlik JR, Mac Fall G, Zea S (2002) Does the odor from sponges of the genus Ircinia protect them from fish predators? J Chem Ecol 28:1103–1115
Piazzi L, Balata D (2008) The spread of Caulerpa racemosa var. cylindracea in the Mediterranean Sea: an example of how biological invasions can influence beta diversity. Mar Environ Res 65:50–61
Piazzi L, Cinelli F (1999) Development and seasonal dynamics of a population of the tropical alga Caulerpa racemosa (Forsskål). J. Agardh in the Mediterranean. Cryptogam Algol 20:295–300
Piazzi L, Ceccherelli G, Cinelli F (2001) Threat to macroalgal diversity: effects of the introduced green alga Caulerpa racemosa in the Mediterranean. Mar Ecol Prog Ser 210:149–159
Piazzi L, Meinesz A, Verlaque M, Akçali B, Antolić B, Argyrou M, Balata D, Ballesteros E, Calvo S, Cinelli F, Cirik S, Cossu A, D’archino F, Djellouli AS, Javel F, Lanfranco E, Mifsud C, Pala D, Panayotidis P, Peirano A, Pergent G, Petrocelli A, Ruitton S, Žuljević A, Ceccherelli G (2005) Invasion of Caulerpa racemosa var cylindracea (Caulerpales, Chlorophyta) in the Mediterranean Sea: an assessment of the spread. Cryptogam Algol 26(2):189–202
Ruitton S, Javel F, Culioli JM, Meinesz A, Pergent G, Verlaque M (2005) First assessment of the Caulerpa racemosa (Caulerpales, Chlorophyta) invasion along the French Mediterranean coast. Mar Pollut Bull 50:1061–1068
Tsoukatou M, Hellio C, Vagias C, Harvala C, Roussis V (2002) Chemical defense and antifouling activity of three mediterranean sponges of the genus Ircinia. Z Naturforsch 57:161–171
Vacelet J (1991) Statut des éponges commerciales en Méditerranée. In: Boudouresque CF, Avon M, Gravez V (eds) Les espèces marines à protéger en Méditerranée. Gis Posidonie publi, France, pp 35–42
Vázquez-Luis M, Sanchez-Jerez P, Bayle-Sempere JT (2008) Changes in amphipod (Crustacea) assemblages associated with shallow-water algal habitats invaded by Caulerpa racemosa var. cylindracea in the western Mediterranean Sea. Mar Environ Res 65:416–426
Verlaque M, Boudouresque CF, Meinesz A, Gravez V (2000) The Caulerpa racemosa complex (Caulerpales, Ulvophyceae) in the Mediterranean Sea. Bot Mar 43:49–68
Verlaque M, Durand C, Huisman JM, Boudouresque CF, Le Parco Y (2003) On the identity and origin of the Mediterranean invasive Caulerpa racemosa (Caulerpales, Chlorophyta). Eur J Phycol 38:325–339
Wang N, Shin J (2008) Secondary metabolites of marine sponge Sarcotragus sp. J Biotechnol 136:577–578
Žuljević A, Antolić B, Onofri V (2003) First record of Caulerpa racemosa (Caulerpales: Chlorophyta) in the Adriatic Sea. J Mar Biol Assoc UK 83:711–712
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10530_2011_43_MOESM1_ESM.mpg
Online Resource – Video 1 Shallow rocky bottom affected by Caulerpa racemosa. Diver is collecting a sponge (Sarcotragus spinosulus) completely overgrown by the invasive alga at a depth of 7 m. Note that diver detaches the invaded sponge only by hand, which is not possible if the sponge is healthy (MPG 8431 kb)
Rights and permissions
About this article
Cite this article
Žuljević, A., Thibaut, T., Despalatović, M. et al. Invasive alga Caulerpa racemosa var. cylindracea makes a strong impact on the Mediterranean sponge Sarcotragus spinosulus . Biol Invasions 13, 2303–2308 (2011). https://doi.org/10.1007/s10530-011-0043-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-011-0043-6