Abstract
Since the 1980s the Ponto-Caspian gammarid Dikerogammarus villosus has spread throughout Europe while displacing native species and is predicted to invade further continents. After it was introduced into Europe in the 1890s the North American crayfish Orconectes limosus spread throughout Europe and served as a vector to displace native crayfish as well. In Lake Constance (Germany) the previously dominant gammarid Gammarus roeselii is subjected to both of these invasive crustaceans. In our experiments both species placed predation pressure on G. roeselii. Kairomone perception tests in a Y-maze revealed the capability of the gammarids G. roeselii and D. villosus to perceive and avoid the scent of the predator crayfish O. limosus. Both species also avoided the kairomone of the other gammarid, but did not avoid the scent of their own species. This taxa specific behavior suggests that taxa specific signals are used. This behavior can help the gammarids avoid shelters previously occupied by predators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive species became one of the major threats of limnic ecosystems in the last decades (Sala et al. 2000). The ecological impact of invasives increased dramatically as European streams became more connected via channels coupled with increased freight traffic on these waterways (Bij de Vaate et al. 2002; Parmesan et al. 2005). These anthropogenous transformed waterways serve as major vectors and targets for invasives. For example now invasive species comprise 90% of the macroinvertebrate counts in the River Rhine (van der Velde et al. 2000; van Riel et al. 2006). Invasions of nonnative species may have positive or negative effects on the native fauna which can easily spread through the food web. For example in Lake Constance (Germany) the introduction and population explosion of the invasive zebra mussel (Dreissena polymorpha) increased the number of wintering waterbirds fourfold because it acts as a food source (Werner et al. 2005). In contrast in North America the invasive opossum shrimp Mysis relicta led to the collapse of Salmon stocks and thus negatively impacted the bald eagle as a top predator (Spencer et al. 1991).
One of the most successful invasive benthic animals in Europe at the moment is the predatory gammarid Dikerogammarus villosus (Sowinsky). It originated in the Ponto-Caspian area and used the Main-Danube channel which opened in 1992 for the dispersal throughout the rest of Europe (Bij de Vaate et al. 2002). D. villosus quickly arrived in Germany (Tittizer et al. 2000), Netherlands (Kelleher et al. 1998), France (Devin et al. 2001) and Italy (Casellato et al. 2006). There it often displaced native amphipods and thus became the dominant amphipod species (Devin et al. 2003; Bollache et al. 2004; van Riel et al. 2006). Different reasons for its success are discussed. D. villosus’ tolerance to high salinity allows its transport via ballast water in ships (Bruijs et al. 2001). Its big size and its high predatory potential on other gammarids enhances its ability to displace competitors (Dick et al. 2002; Devin et al. 2003; Kinzler and Maier 2003; Bollache et al. 2004). It even can outcompete Gammarus tigrinus which originated in North America and displaced many European gammarids before the arrival of D. villosus (Haas et al. 2002). Therefore, D. villosus could be a threat for North American limnic ecosystems as well such as the Great Lakes system (Bruijs et al. 2001). In Lake Constance (Germany) D. villosus was first recorded in 2002. In comparison to the River Rhine, Lake Constance was invaded later by most of the neozoans. Potential reasons are the 10 m high water falls (Rheinfall) at Schaffhausen and the intact habitat structures in the upper Rhine river (Uehlinger et al. 2009).
Another invasive benthic crustacean in Lake Constance is the omnivorous North American crayfish Orconectes limosus (Rafinesque). It was introduced in this lake in the late 1980s and has now spread over the whole littoral zone (Hirsch et al. 2008). This species was originally introduced into Germany in 1890 to replace the native crayfish stock after the latter were reduced by accidental introduction of crayfish pest (Aphanomyces astaci). Orconectes limosus acted as a major vector for the further dispersal of the crayfish pest (Schweng 1973). In general omnivorous crayfish have had a strong impact on the whole food web, since they consume from nearly every trophic level of the littoral community (Lodge et al. 2000; Bernot and Turner 2001).
Prior to 2002 the dominant gammarid species in Lake Constance was Gammarus roeselii (Gervais). Originating from the Balkans (Jazdzewski 1980), G. roeselii was first described in the surrounding waters of Paris by Gervais (Gervais 1835). It established itself long ago and is in balance with its environment (Josens et al. 2005). G. roeselii established itself in Lake Constance prior to 1974, during the eutrophication of the lake (Hartmann 1977). Therefore, it coexisted with O. limosus for at least one decade. In contrast, the densities of G. roeselii in Lake Constance were strongly reduced after the invasion of D. villosus (Mörtl et al. 2005) most probably due to direct predation. Dick et al. (2002) first described intra-guild predation of D. villosus on several gammarid species. This was later tested on G. roeselii in one-on-one small-scale experiments by Kinzler and Maier (2003).
Since predation is discussed as a main factor for the success of, at least, D. villosus we analyzed the predation pressure of both invasive species (D. villosus and O. limosus) on G. roeselii in aquarium experiments under more natural circumstances with gammarid groups and substrate.
Furthermore, we were interested in predation avoidance strategies of the earlier established G. roeselii. Many animals perceive kairomones from potential predators and use this information to elicit an appropriate reaction (Dodson et al. 1994; Kats and Dill 1998). It is known that G. roeselii can perceive and avoid the kairomones of burbot (Lota lota) and crucian carp (Carassius carassius), but not of Eurasian Perch (Perca fluviatilis) in Lake Constance (Baumgärtner et al. 2002). Overall, different Gammarus species do not have to rely on vision to sense the presence of predatory fish and hence reduce their activity (Holomuzki and Hoyle 1990; Wudkevich et al. 1997). Gammarus pulex reduces its drift when confronted with brown trout (Salmo trutta), the same goes for Gammarus pseudolimnaeus when confronted by rainbow trout (Oncorhynchus mykiss) (Williams and Moore 1982; Andersen et al. 1993).
Therefore, we analyzed the perception and possible avoidance of G. roeselii and D. villosus regarding kairomones from crayfish as well as from both gammarid species.
Materials and methods
For all experiments we used individuals of G. roeselii and D. villosus from the littoral zone of Lake Constance near the city of Constance that not has been used in prior experiments. They were acclimatized for at least 24 h at 14°C in fresh, filtered (40 μm) lake water. The light regime in the utilized climate chamber was 14 h light and 10 h dark. Intact male specimens of the crayfish O. limosus were caught in the western part of the lake and kept under the same conditions. The carapax length was 4.0 (±0.3) cm, measured from the tip of the rostrum along the median line to the posterior boundary of the carapax. Their wet weight was 20.4 (±4.8) g. All experiments were conducted between March and July 2006.
Predation experiments
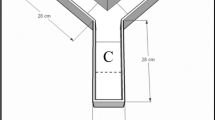
The predation experiments were carried out in aquaria (44 × 22 × 25 cm) equipped with eight stones [10.3 (±10.7) cm ∅], and a 2 cm sand layer [489 (±178) g]. One O. limosus per aquarium was introduced representing the crayfish predator. All five replicate experiments were conducted with 30 gammarids per aquarium. We analyzed the predation of O. limosus on G. roeselii and D. villosus separately. The predation rates between both gammarid species were tested with 15 individuals of each species per aquarium. Therefore, the gammarid density remained at 30 individuals per aquarium. As a control we used five aquaria with 30 individuals of only one gammarid species to obtain specific mortality and cannibalism rates.
Prior to beginning the experiment crayfish were not fed for 72 h and were allowed to adapt to the aquaria for 24 h. The experiments were run at 14°C beginning with the addition of gammarids and ended after 72 h with the counting of remaining gammarids. Only individuals that were completely gone were counted as predated.
Kairomone perception
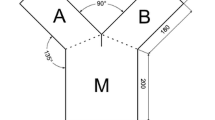
To analyze the reactions of gammarids in the presence of kairomones we used a Y-maze according to Baumgärtner et al. (2002) (Fig. 1). The maze was inclined at 2.5°, the inflow outlets were attached at 7 cm height. The previously prepared kairomone containing water and pure lake water were added separately through arms A and B at a constant flow of 0.5 l/min, and the use of arm A or B for the kairomone treated water was switched after every run. The temperature of the water was kept between 13.5 and 14.5°C. Homogenous indirect light conditions were established and controlled daily via a light metering device.
The water containing kairomones was produced while the different animals were kept in basins with 40 l fresh and filtered (40 μm, gauze) lake water. The aquaria were well aerated during the entire time of incubation. We used 180 gammarids or 5 O. limosus per basin with incubation times between 24 and 72 h. To avoid contamination of the water with particles the animals were not fed during the incubation and 24 h prior to that. We observed no loss of gammarids during the incubation time of the kairomone. Therefore, we could exclude presence of exudates from gammarid carcasses. In addition the kairomone containing water was filtered (40 μm, gauze). Between the runs the Y-maze was cleaned with 60°C water. A flow was created of 0.5 l min−1 in each of both arms, which means 1 l min−1 in the mixing zone, This was kept constant and controlled.
After allowing the flow system to stabilize at an actual flow of 15.3 cm min−1 for at least 2 min, 20 gammarids per run were placed in the mixing zone. The gammarid numbers in the different areas of the Y-maze (arm A and B, mixing zone) were counted every 2 min for half an hour. We analyzed the mean densities for every run from 10 to 30 min because the gammarids needed a few minutes to disperse in the Y-maze. In all treatments we controlled activity of the gammarids over the whole experiment time.
In the first experiment we analyzed the ability of different gammarid species to scent each other in water containing kairomone from the opposite species. This water contained the kairomones from each of those species separately which was incubated for 24 h. As an additional treatment we acclimatized D. villosus to the scent of the prey G. roeselii 1 week prior to the experiment by feeding it only living G. roeselii. In the second experiment we tested the reaction of both gammarid species to the kairomone of O. limosus. To test the possible different reactions to different concentrations of kairomone we incubated the crayfish in water for 24, 36, 48 and 72 h to collect the kairomone. There were seven replicates per treatment in both experiments.
As a control we observed the distribution of gammarids in the maze using pure lake water in both arms for G. roeselii and D. villosus separately with 15 replicates each. To test for eventual effects of small temperature changes during the experiment we tested the distribution of gammarids in water between 13.5 and 14.5°C with seven replicates each.
Statistics
All statistical analyses were conducted with StatSoft Statistica 6.0 for Microsoft Windows. All datasets were successfully controlled for homogenous variances with a Levene test.
Predation experiments
The predation rates were calculated as difference in loss of gammarid individuals during the predation experiment itself and the corresponding cannibalism rates from the controls. Differences in the predation or cannibalism rates between the two gammarid species were compared using t-tests in the following cases: predation by O. limosus, predation by the other gammarid species, or cannibalism in the controls. For the predation rates between both gammarid species the rates of predation in the controls were divided by two since the controls were run with 30 individuals of one species and the predation rate between both gammarid species were run with 15 individuals per species.
Kairomone perception
To obtain the preferences of gammarids for one of the two different given water qualities we compared the individual numbers in the corresponding arms with t-tests. Controls were treated likewise for the tested parameter. The significance level (α = 0.05) of the experiment testing an influence of the kairomone from the crayfish on gammarids was adjusted with a sequential Bonferroni correction for the four analyzed incubation times.
Results
Predation experiments
Our experiments revealed significant differences in predation rates by O. limosus on the two gammarid species (Table 1). The rates of predation on G. roeselii were 2.5 times higher than those on D. villosus. The predation between the gammarid species was highly asymmetric and 6.5 times higher from D. villosus on G. roeselii than vice versa (Table 1). The cannibalism rates in the control for gammarids did not differ significantly between the two species (Table 1).
Kairomone perception
Both gammarid species could perceive the kairomone of the respective other species. All preferred pure lake water to water containing the respective foreign kairomone. The behavior of the individuals of D. villosus acclimatized to the scent of G. roeselii was the same as it was for the unacclimatized ones (Fig. 2a: G. roeselii, t = 4.375, n = 5, P = 0.002; Fig. 2b: D. villosus, t = 4.815, n = 5, P = 0.001; ‘trained’, t = 3.245, n = 5, P = 0.012; t-test). In contrast, both gammarid species did not avoid water containing kairomone originated from their own species (Fig. 2a: G. roeselii, t = 0.157, n = 5, P = 0.879; Fig. 2b: D. villosus, t = 0.527, n = 5, P = 0.623; t-test).
Reaction of a G. roeselii and b D. villosus regarding the kairomone of both gammarid species. Numbers of individuals in different sections of a Y-maze with different waters (means ± SE). */*** significant t-test between the test waters after sequential Bonferroni (P ≤ 0.5, ≤0.001; n = 7). See text for further details
Both tested gammarid species showed different reactions toward the kairomone of O. limosus. There was a significant avoidance of G. roeselii to crayfish kairomone after 48 and 72 h incubation time (Fig. 3a; Table 2). The low number of individuals in the pure lake water and high number in the mixing zone at 72 h incubation time can be explained by inactivity. In this single treatment most of the gammarids were complacent in the mixing zone not moving from where they were inserted. We interpret this reaction as a fear response due to a high kairomone concentration in the water. The reaction of D. villosus did not seem to be influenced by the incubation time of the kairomone. There was a significant avoidance by it to the scent of kairomone from the 24 and 48 h incubations, but not for 36 and 72 h (Fig. 3b; Table 2).
Reaction of a G. roeselii and b D. villosus regarding kairomones of O. limosus. The incubation time O. limosus in lake water for test water production was varied. Numbers of individuals in different sections of a Y-maze with different waters (means ± SE). **/*** significant t-test between the test waters after sequential Bonferroni (P ≤ 0.1, ≤ 0.001; n = 7). See text for further details
The controls showed no preference for either of the arms of the Y-maze (Table 2). Further more, both gammarids had no water temperature preference for slightly cooler (13.5°C) or warmer (14.5°C) water (G. roeselii: t = 1.241, n = 7, P = 0.238; D. villosus: t = 0.852, n = 7, P = 0.411). Most of the gammarids in all treatments moved about actively in the Y-maze during the whole experiment. The inactivity of G. roeselii in the presence of the kairomone of O. limosus from the 72 h incubation was the only exception.
Discussion
With these experiments we confirmed predation of the invasive crustaceans D. villosus and O. limosus on the local gammarid G. roeselii. The presence of D. villosus represents a real menace for G. roeselii. The impact of predation by one adult crayfish was only three times higher than that of 15 D. villosus on G. roeselii. Asymmetric predation of D. villosus on G. roeselii in small-scale experiments has been cited in previous literature (Kinzler and Maier 2003) and could be confirmed by us for more natural systems. The predation pressure on G. roeselii in Lake Constance is exacerbated by the asymmetric higher predation of the crayfish O. limosus on G. roeselii than on D. villosus. This can be explained by the higher activity of G. roeselii outside its shelters when compared with that of D. villosus (Kinzler and Maier 2006; van Riel et al. 2007). In both gammarid species we observed cannibalism in the control experiment. With our setup we cannot distinguish whether it was predation or necrophagy.
The kairomone experiments revealed the capability of G. roeselii to perceive the scent of D. villosus and O. limosus and react appropriately by avoiding high risk areas thereby reducing its predation pressure. D. villosus showed a similar behavior to the scent of G. roeselii. In this case avoidance is a negative reaction for D. villosus since G. roeselii represents the prey. This reaction can be observed even when D. villosus has used G. roeselii as single food source for 1 week. Most probably D. villosus just avoids all unfamiliar crustaceans. Because of its evolution in the Ponto-Caspis, D. villosus is not familiar to foreign gammarids as prey. Over the last 5 years after its introduction, D. villosus still appears incapable of adapting its responses to kairomones from its new prey in Lake Constance. To gain deeper insight into this behavior analyzes of kairomones emitted by other crustacean species also originating from the Ponto-Caspis like Dikerogammarus haemobaphes or Echinogammarus spp. should be carried out. Repeating our experiments in several years from now might show that D. villosus is capable of adapting this behavior to the Middle European gammarids in the long term.
From our results we deducted some characteristics of the infochemicals used by both gammarid species. First, the signal must consist of different substances or substance combinations for different taxa. Both gammarids are able to distinguish between the scent of their own and other species. Second, we figure the kairomone is to a degree water resistant over the time studied. We interpret the reaction of G. roeselii toward the scent of O. limosus as an intensifying reaction to increasing concentrations of the kairomone until they eventually retreated into a corner and remained motionless. If concentrations of kairomone can increase over the whole range of analyzed kairomone releasing times then kairomone as a substance must be stable for at least 72 h in water. Thirdly, kairomone cannot be very volatile and must be chemically stable in well oxygenated environments because the aquaria were well aerated during the entire time of incubation.
To translate our results to the field situation it is important to consider the conditions of kairomone release. To achieve our findings we used longer incubation times than would occur in streams or the littoral zone of a lake with wave impact. Therefore, the observed behavior of gammarids is especially applicable for areas with low water velocities such as under stones or in the interstitial zone. We do not think that these kairomones are used to perceive and escape an approaching predator, rather they could be used to choose which shelter to occupy i.e. under a stone that is already occupied by foreign species (and most probably a predator) or by the same species (and most probably a friend). This hypothesis is supported by our field observations. Even when both gammarid species occur in the same area we always find each species segregated to its own stone.
References
Andersen TH, Friberg N, Hansen HO et al (1993) The effects of introduction of brown trout (Salmo trutta L.) on Gammarus pulex—drift and density in 2 fishless Danish streams. Arch Hydrobiol 126:361–371
Baumgärtner D, Jungbluth AD, Koch U et al (2002) Effects of infochemicals on microhabitat choice by the freshwater amphipod Gammarus roeseli. Arch Hydrobiol 155:353–367
Bernot RJ, Turner AM (2001) Predator identity and trait-mediated indirect effects in a littoral food web. Oecologia 129:139–146
Bij de Vaate A, Jazdzewski K, Ketelaars HAM et al (2002) Geographical patterns in range extension of Ponto-Caspian macroinvertebrate species in Europe. Can J Fish Aquat Sci 59:1159–1174
Bollache L, Devin S, Wattier R et al (2004) Rapid range extension of the Ponto-Caspian amphipod Dikerogammarus villosus in France: potential consequences. Arch Hydrobiol 160:57–66
Bruijs MCM, Kelleher B, van der Velde G et al (2001) Oxygen consumption, temperature and salinity tolerance of the invasive amphipod Dikerogammarus villosus: indicators of further dispersal via ballast water transport. Arch Hydrobiol 152:633–646
Casellato S, La Piana G, Latella L et al (2006) Dikerogammarus villosus (Sowinsky, 1894) (Crustacea, Amphipoda, Gammaridae) for the first time in Italy. Ital J Zool 73:97–104
Devin S, Beisel JN, Bachmann V et al (2001) Dikerogammarus villosus (Amphipoda: Gammaridae): another invasive species newly established in the Moselle river and French hydrosystems. Ann Limnol-Int J Limnol 37:21–27
Devin S, Piscart C, Beisel JN et al (2003) Ecological traits of the amphipod invader Dikerogammarus villosus on a mesohabitat scale. Arch Hydrobiol 158:43–56
Dick JTA, Platvoet D, Kelly DW (2002) Predatory impact of the freshwater invader Dikerogammarus villosus (Crustacea: Amphipoda). Can J Fish Aquat Sci 59:1078–1084
Dodson SI, Crowl TA, Peckarsky BL et al (1994) Nonvisual communication in fresh-water benthos—an overview. J N Am Benthol Soc 13:268–282
Gervais M (1835) Note sur deux espèces de Crevettes qui virent aux environs de Paris. Ann Sci Nat 2:127–128
Haas G, Brunke M, Streit B (2002) Fast turnover on dominance of exotic species in the Rhine river determines the biodiversity and ecosystem function: an affair between amphipods and mussels. In: Leppäskoski E, Gollasch S, Olenin S (eds) Invasive aquatic species of Europe. Kluwer, Dordrecht, pp 426–432
Hartmann J (1977) Burbot (Lota lota) in eutrophicated lake of Constance. Arch Hydrobiol 80:360–374
Hirsch P, Nechwatal J, Fischer P (2008) A previously undescribed set of Saprolegnia spp. in the invasive spiny-cheek crayfish (Orconectes limosus, Rafinesque). Fund Appl Limnol 172:161–165
Holomuzki JR, Hoyle JD (1990) Effect of predatory fish presence on habitat use and diel movement of the stream amphipod, Gammarus minus. Freshw Biol 24:509–517
Jazdzewski K (1980) Range extension of some gammaridean species in European inland waters caused by human activity. Crustac Suppl 6:84–107
Josens G, Bij de Vaate A, Usseglio-Polatera P et al (2005) Native and exotic Amphipoda and other Peracarida in the River Meuse: new assemblages emerge from a fast changing fauna. Hydrobiologia 542:203–220
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394
Kelleher B, Bergers PJM, van den Brink FWB et al (1998) Effects of exotic amphipod invasions on fish diet in the lower Rhine. Arch Hydrobiol 143:363–382
Kinzler W, Maier G (2003) Asymmetry in mutual predation: possible reason for the replacement of native gammarids by invasives. Arch Hydrobiol 157:473–481
Kinzler W, Maier G (2006) Selective predation by fish: a further reason for the decline of native gammarids in the presence of invasives? J Limnol 65:27–34
Lodge DM, Taylor CA, Holdich DM et al (2000) Nonindigenous crayfishes threaten North American freshwater biodiversity: lessons from Europe. Fisheries 25:7–20
Mörtl M, Mürle U, Ortlepp J et al (2005) Dikerogammarus villosus (Crustacea: Amphipoda) und Corbicula fluminea (Bivalvia: Veneroidea) im Bodensee. Institut für Seenforschung 5:15–30
Parmesan C, Gaines S, Gonzalez L et al (2005) Empirical perspectives on species borders: from traditional biogeography to global change. Oikos 108:58–75
Sala OE, Chapin FS, Armesto JJ et al (2000) Biodiversity—global biodiversity scenarios for the year 2100. Science 287:1770–1774
Schweng E (1973) Orconectes limosus in Deutschland insbesondere im Rheingebiet. Freshw Crayfish 1:79–87
Spencer CN, Mcclelland BR, Stanford JA (1991) Shrimp stocking, salmon collapse, and eagle displacement. Bioscience 41:14–21
Tittizer T, Schöll F, Banning M et al (2000) Aquatische Neozoen im Makrozoobenthos der Bundeswasserstraßen Deutschlands. Lauterbornia 39:1–72
Uehlinger U, Wantzen KM, Leuven RSEW (2009) Rhine river basin. In: Tockner K, Uehlinger U, Robinson C et al (eds) The rivers of Europe. Elsevier, London, pp 199–245
van der Velde G, Rajagopal S, Kelleher B et al (2000) Ecological impact of crustacean invaders: general considerations and examples from the Rhine river. Crustac Issues 12:3–33
van Riel MC, van der Velde G, Rajagopal S et al (2006) Trophic relationships in the Rhine food web during invasion and after establishment of the Ponto-Caspian invader Dikerogammarus villosus. Hydrobiologia 565:39–58
van Riel MC, Healy EP, van der Velde G et al (2007) Interference competition among native and invader amphipods. Acta Oecol 31:282–289
Werner S, Mörtl M, Bauer HG et al (2005) Strong impact of wintering waterbirds on zebra mussel (Dreissena polymorpha) populations at Lake Constance, Germany. Freshw Biol 50:1412–1426
Williams DD, Moore KA (1982) The effect of environmental-factors on the activity of Gammarus pseudolimnaeus (Amphipoda). Hydrobiologia 96:137–147
Wudkevich K, Wisenden BD, Chivers DP et al (1997) Reactions of Gammarus lacustris to chemical stimuli from natural predators and injured conspecifics. J Chem Ecol 23:1163–1173
Acknowledgments
This work was supported by the Sonderforschungsbereich Bodenseelitoral (CRC 454) of the Deutsche Forschungsgemeinschaft. The authors would like to thank Stefan Werner and all members of the ANEBO group for fruitful discussions on the recent invaders in Lake Constance. We thank Sonja Raub for correcting the English and style.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hesselschwerdt, J., Tscharner, S., Necker, J. et al. A local gammarid uses kairomones to avoid predation by the invasive crustaceans Dikerogammarus villosus and Orconectes limosus . Biol Invasions 11, 2133–2140 (2009). https://doi.org/10.1007/s10530-009-9492-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-009-9492-6