Abstract
A key gap in understanding the long-term success of invasive species is how biotic interactions change with the duration of experience in the introduced range. We examined biotic interactions using a common garden experiment with native, hybrid, and exotic Senecio species representing a range of experience in the UK. Introduced species had fewer aphids and pathogens and more root colonization by mycorrhizal fungi compared to natives; hybrids generally had intermediate levels of interactions. The duration of experience in the introduced range was reflected by an increasing degree of variability in enemy release. These findings support the enemy release hypothesis and indicate the potential for changes in enemy release as time and experience in the new range increase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding why some invasions are successful is an important aspect of both basic community ecology and management of a global threat to biodiversity and ecosystem services (Mack et al. 2000; Pimentel et al. 2000, 2001). Compared to native plants, exotics may display advantages that can increase the likelihood of successfully establishing and reproducing in the new range (Hawkes 2007). Examples are release from natural enemies (Elton 1958), increased size or competitive ability (Blossey and Notzold 1995), novel weapons (Callaway and Ridenour 2004), and mutualist facilitation (Richardson et al. 2000), among others (reviewed by Mitchell et al. 2006). The ability to identify some successful invaders based on life history traits (e.g., Rejmanek et al. 1995; Rejmanek and Richardson 1996) suggests that species can be preadapted to succeed in new habitats. Yet both the lag sometimes observed between arrival and expansion of invaders and the occurrence of multiple introductions before invasive spread imply that post-introduction evolutionary changes also affect the invasion process (Ellstrand and Schierenbeck 2000).
Evolutionary changes in exotics after arrival in a new habitat are likely to be an important component of both invasion success and impacts (Strayer et al. 2006). Invading plants can undergo evolution as a result of founder effects, hybridization, and adaptation in the new environment (Bossdorf et al. 2005; Ellstrand and Schierenbeck 2000; Sakai et al. 2001). Biotic interactions can also act as a strong selection pressure in the new range, with differences in the intensities of interspecific interactions in different communities producing a geographic mosaic of potential coevolution (Thompson 1999). Berenbaum and Zangerl (2006) found that the likelihood of reciprocal ecological interactions between wild parsnip and parsnip webworms decreased with increased trophic complexity in the form of alternate host plants and webworm parasitoids in both their native and introduced ranges. Other studies have found differences in the strength of coevolutionary interactions due to the presence or absence of competitors, herbivores, pathogens, parasites, pollinators, and other biota (e.g., Benkman et al. 2008; Berenbaum and Zangerl 2006; Burdon and Thrall 1999; Kraaijeveld and Godfray 1999; Thompson and Cunningham 2002). Local success and impacts of invasive plants may partly result from whether the novel range represents a coevolutionary ‘hot’ spot where interactions are strong or a ‘cold’ spot where interactions are weak (Thompson 2005).
The development of multiple simultaneous biotic interactions (or the lack thereof), while difficult to tease apart, contributes to the success of initial invasions and the long-term persistence of invasiveness. Naïve introduced species in a new habitat may escape negative interactions or, conversely, native species in the presence of a new invader may lack resistance to negative interactions. The role of interactions in the success of invasive species has been supported through biogeographical comparisons of conspecifics, with escape from negative interactions in invaders that are considered noxious or invasive (Hawkes 2007).
However, naïve species are unlikely to remain naïve indefinitely and interactions may develop through morphological, physiological, behavioral, or life history trait changes (e.g., Carroll 2007; Strauss et al. 2006). How quickly reciprocal interactions develop may vary locally depending on community structure and selective pressure (Thompson 2005). In a meta-analysis of studies quantifying herbivore and pathogen damage, Hawkes (2007) found that recently introduced species had less damage relative to those in residence for longer periods in both biogeographic and community comparisons. Other studies have demonstrated that the intensity of interactions is lower for co-occurring individuals compared to those that have been geographically separated (e.g., Callaway et al. 2005). Understanding whether and how increasing experience in the introduced habitat alters biotic interactions could help us to pinpoint fundamental differences between native and introduced species and to understand the transition between invasive and non-invasive exotics.
We hypothesized that both origin (native vs. exotic) and differences in the duration of post-introduction experience in the local habitat would be reflected in the relative abundance and impacts of both positive and negative biotic interactions. We may expect, for example, more escape from negative interactions in exotic vs. native species, and more so in naïve compared to experienced exotic species. We tested this idea using a common garden design and a group of four congeneric species. We chose species in the genus Senecio (Asteraceae) to represent a cline of experience in the local habitat while minimizing phylogenetic variation. The species were: (1) S. vulgaris, a native plant, (2) S. squalidus, a species originating allopatrically in the UK after its hybrid parent was introduced from Sicily before 1702 (Harris 2002; James and Abbott 2005), (3) S. vulgaris × S. squalidus, a hybrid which arises naturally and repeatedly in the UK where both species are found (e.g., Lowe and Abbott 2003), and (4) S. ciliocarpa Dazzler, a cultivar introduced to the UK in 1998 (B. Sims, Thompson and Morgan Ltd., personal communication), but not yet escaping from garden landscapes. We also included populations of S. vulgaris grown from seed collected in California, where it was introduced ca. 130 years ago (CalFlora 2003). Growth, reproduction and biotic interactions of these species, which are closely related (e.g., Comes and Abbott 2001; Pelser et al. 2002), were followed for two consecutive years.
We tested the importance of origin and local experience by comparing all four species (S. vulgaris, native most experienced; S. squalidus, exotic present for several hundred years; S. vulgaris × S. squalidus, exotic-native hybrid less experienced than both parental lines; S. ciliocarpa, exotic recently introduced and most naïve). In addition, we used the native-exotic hybrid (S. vulgaris × S. squalidus) to distinguish effects of local experience from genetic factors; and, to understand the potential for loss of experience, we examined biotic interactions of S. vulgaris reintroduced to the UK from a part of its introduced range in California. We predicted that naïve exotics would have the least damage from pests and pathogens, consistent with the enemy release hypothesis, and that the intensity of enemy damage would increase with local experience. If greater experience in the local habitat increases susceptibility to enemies, then more damage should occur on the natives and S. squalidus, a long-standing and successful invader, compared to the recently introduced S. ciliocarpa. The most abundant enemies of Senecio are fungal pathogens, and their impact on plant fitness was tested through the application of fungicide, with the expectation that exotics would benefit least from this treatment. If genetic factors are more important than local experience, then the hybrid S. vulgaris × S. squalidus should be intermediate in all other respects; whereas if local experience matters more than genetic factors the hybrid should behave like a newly introduced species. These comparisons further our understanding of the importance of biotic interactions for growth and reproduction of invasive species.
Materials and methods
Experimental design and species
The common garden was set up in the Walled Garden at the University of York, UK. We used a randomized complete blocks design with four blocks, each block containing two plots (0.5 m × 0.5 m) per plant species—one control plot and one treated with fungicide.
Seeds of S. vulgaris were collected from both the US (pooled from five populations in Berkeley and Oakland, CA) and the UK (from one population at the U. York campus), seeds of S. squalidus and S. vulgaris × S. squalidus were collected in York, and S. ciliocarpa seeds were provided by Thompson and Morgan Ltd. (Ipswich, UK). The S. vulgaris populations are referred to as ‘native’ and ‘returned’ to the UK, S. vulgaris × S. squalidus as a hybrid, and S. ciliocarpa and S. squalidus as exotic. Seeds were germinated in the greenhouse and added to plots as 2-week-old seedlings on May 9, 2003 and May 24, 2004. Plots received the same treatments and species in both years. The common garden was weeded as necessary.
Fungicide was used to reduce biotic interactions. In 2003, a single dose of benlate (50%, Dupont, an inhibitor of fungal microtubule assembly) was applied to soils at the manufacturer’s recommended dose of 0.1 g m−2, sprayed evenly onto soils on May 7 before seedlings were transplanted. We added aboveground fungicide application (1.5 ml per plant of 0.06% myclobutanil solution, a systemic fungicide inhibiting ergosterol biosynthesis) in 2004 and increased the soil (benlate) application to 5 g m−2, because of substantial pathogen attack in 2003. Fungicides were applied twice during 2004—benlate on May 28 and July 5, myclobutanil on June 22 and July 4. Control plots received an equal volume of water. Myclobutanil is recommended for control of rusts and powdery mildews and benlate reduces growth of a wide range of soil fungi, including pathogens, lichens, and mycorrhizal fungi (Fitter and Nichols 1988; Kahiluoto and Vestberg 2000; Newsham et al. 1995; Smith et al. 2000).
Plant size, growth, reproduction, and survival
Plants were harvested in early-July 2003 and mid-July 2004, after ~8 weeks growth. Prior to harvest, plant height, width, and the numbers of leaves, flowers, and seed heads were recorded. Plants were excavated, roots washed, shoots and roots oven-dried at 60°C for 5 days, and weighed. In 2004, plant buds were abundant at the harvest and were counted to reflect potential flowering. The number of seeds per flower in 2003 was determined for ten mature flower heads per species (each randomly selected from a different plant).
Relative growth rates (RGR) were calculated based on biomass over time. Biomass was determined allometrically from plants harvested sequentially throughout the growing season to capture a range of plant sizes. Stepwise multiple regression best predicted biomass by the number of leaves (biomass = (0.066 × leaves) + 0.654, r 2 = 0.65, P < 0.001). Time point measurements were used to calculate RGR as [ln(W 2) − ln(W 1)]/(t 2 − t 1) where W = biomass, t = time, and the subscripts indicate the first and second measurements. RGR was calculated based on two dates for the natives (May 29 and June 29, 2003) and an additional third date for the exotics (July 16, 2003) to capture their entire range of growth (N = 30–40).
Biotic interactions
In 2003, aboveground pathogens were measured in June for S. vulgaris and S. vulgaris × S. squalidus, and in mid-July for S. squalidus and S. ciliocarpa, which were slower growing. In 2004, pathogens were evaluated in late July. Pathogen abundance was quantified as the proportion of infected leaves out of the total number of leaves on the plant, which is indicative of the overall impact and allows for comparisons among species. Infection was diagnosed visually by the presence of lesions. The area of each leaf infected was similar and typically reached >75% across all species except S. ciliocarpa, which typically had a lower amount of leaf area infected.
The abundance of insects on the plants was measured each year. Aphids were evaluated at their peak abundance on each species in mid-June 2003 and late-June/early-July 2004. We scored aphids at three levels: none (0), on <50% of leaves/stems (1), or on >50% of leaves/stems (2). Other insects and damage observed on the plants were also recorded.
To understand the impact of aphids on plants, we measured aphid honeydew production to estimate the magnitude of photosynthetically-fixed carbon diverted to aphids. Honeydew production was quantified in control plots when aphid populations peaked in 2004 (late-June/early-July), and S. ciliocarpa was excluded from this analysis because it bore very few aphids. Mesh cages (2.5 cm diam.) holding pre-weighed aluminum foil disks were clamped onto the underside of leaves to collect honeydew over 24 h. The disks were air dried for 24 h and reweighed.
Belowground interactions with soil nematodes and root fungi were measured in 2003 in control plots only. Soil nematodes were quantified as described in Whitehead and Hemming (1965). For root length colonization by arbuscular mycorrhizal (AM) fungi and root pathogens, fresh roots of three plants per species were stained with acid fuchsin (Koske and Gemma 1989) and aseptate and septate hyphae were quantified with microscopy using the magnified intersections method (100 intersections) (McGonigle et al. 1990).
Statistical analyses
Data were analyzed with ANOVA (SPSS 2005), with block as a random factor and species and fungicide treatment as fixed factors. Posthoc comparisons (Ryan-Einot-Gabriel-Welsch F test) were made when P < 0.05. ANOVAs for root colonization were run without block because the distribution of surviving plants at the end of the growing season was unbalanced. Proportion data were arc sine transformed. Aphids, which were quantified categorically, were analyzed as a function of block, species, and fungicide with non-parametric Kruskal–Wallis H tests and posthoc tests for significant factors were run as pairwise comparisons with Mann–Whitney U tests. Correlations among the dependent variables were examined with Pearson’s r or Spearman’s rho. Untransformed data are reported as means ± 1SD; transformed data are reported as means ± asymmetric 95% CI.
Results
Plant size, growth, reproduction, and survival
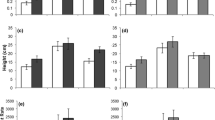
In 2003, S. squalidus and S. vulgaris × S. squalidus were larger aboveground than the natives and S. ciliocarpa (Fig. 1a). In both years, the naïve exotic S. ciliocarpa plants were smaller aboveground than other species (Fig. 1a). Belowground biomass was greatest in the exotic S. squalidus in both years, though there was a large degree of overlap with other species in 2004 (Fig. 1b). Fungicide did not affect root biomass in 2003 but did in 2004 (F 4, 27 = 4.91, P = 0.035), with 25% more root biomass in fumigated plots. RGR in 2003 differed significantly across species (Table 1), but was not affected by fungicide. The exotic S. ciliocarpa grew significantly more slowly and the hybrid S. vulgaris × S. squalidus grew more rapidly than other species (Table 1).
Plant biomass (a) aboveground and (b) belowground in 2003 and 2004 (N = 8). In both years, biomass among species varied significantly aboveground (2003: F 4, 27 = 14.64, P < 0.001; 2004: F 4, 27 = 11.26, P < 0.001) and belowground (2003: F 4, 27 = 17.11, P < 0.001; 2004: F 4, 27 = 3.47, P = 0.021). Different letters indicate significant differences among species in 2003 (a–c) and 2004 (x, y)
The exotics had about twice as many seeds per flower (Table 1), but produced 10–20 times fewer flowers compared to S. vulgaris (Fig. 2), with the hybrid intermediate between the native and exotic species. Flower production by the native S. vulgaris exceeded that of the returned population by 30–55% in 2004, but these differed by less than 10% in 2003 (Table 1; Fig. 2).
Fungicide application strongly affected flower production with 25% more flowers in 2004 (Fig. 2), but not in 2003. Other factors did not explain plant flowering in either year. Aboveground biomass explained only 19% of the variation in flower production in both 2003 (P < 0.001) and 2004 (P = 0.004).
Because the timing of flowering differed among species, in 2004 we also measured flowers that had not yet opened or had gone to seed (Table 1). Species differed in both buds and seed heads. Overall, S. vulgaris (native and returned) had very similar numbers of flower buds, but six times more seedheads than either the exotic S. squalidus or the hybrid. In contrast, the naïve S. ciliocarpa flowered later than the other species, with no buds, flowers, or seed heads at harvest.
Plant survival was generally high (>92%) across all species. Fungicide decreased early deaths in 2003 by 6% (F 1, 27 = 13.81, P = 0.001). Among species, death due to stem rot may have been lower in the hybrid S. vulgaris × S. squalidus compared to all other species (P = 0.054; Table 1). Neither block (P = 0.65) nor the interaction of species with fungicide (P = 0.36) affected plant survival.
Biotic interactions
The primary aboveground interactions detected were with aphids and leaf pathogens. Three species of aphids were observed on the plants in both years: Brachycaudus cardui, Macrosiphum rosae, and Aphis fabae. The aphid species datasets are pooled because their distribution across plants did not vary (data not shown). Total aphid abundance was lower on exotics compared to native, returned, and hybrid Senecio in 2003 (H = 20.63 P = 0.006), and there were fewer aphids on S. ciliocarpa in 2004 (H = 10.77, P = 0.029) (Fig. 3). Block, fungicide, and the interaction of species with fungicide did not affect aphid abundance in either year. Total plant biomass explained only 0.9% (P = 0.557) and 16% (P = 0.010) of aphid abundance in 2003 and 2004. Aphid honeydew production (0.63 ± 0.67 mg day−1) did not differ significantly across plant species on a per unit leaf area basis (P = 0.571) or across blocks (P = 0.147).
Two fungal pathogens were dominant in this system: Puccinia lagenophorae (Uredinales, Basidiomycota) and Albugo tragopogonis (Albuginales, Oomycota), and there was a brief outbreak of stem rot in 2003 during a period of high rainfall. Pathogen infection was easily diagnosed by the appearance of lesions (orange for P. lagenophorae, white for A. tragopogonis) or rotted stems. Incidence of the two dominant pathogens was significantly positively correlated in 2003 (r 2 = 0.56, P < 0.001), but not in 2004 (r 2 = 0.08, P = 0.09). Pathogen incidence was unrelated to aphid abundance in 2003, while in 2004 aphid abundance and incidence of P. lagenophorae were weakly correlated (r 2 = 0.16, P = 0.01).
Compared to the exotics, the native and hybrid species generally had a greater proportion of their leaves infected by P. lagenophorae (P < 0.001) and A. tragopogonis (P < 0.002), usually greater by 90% or more, though with some variation across years (Fig. 4). The degree of P. lagenophorae infection followed this pattern, but S. squalidus infection increased by 20% in 2004 to levels similar to those found on the hybrid and returned S. vulgaris (Fig. 4a). Infection by A. tragopogonis was significantly more extensive on the hybrid and both native and returned S. vulgaris compared to the exotics (>84 vs. <4%) in both years (Fig. 4b). The naïve exotic S. ciliocarpa consistently had the lowest infection rates for pathogens (0–3% of leaves infected). Plant biomass was not significantly correlated with A. tragopogonis infection in 2003 (P = 0.416) or 2004 (P = 0.238), but for P. lagenophorae infection, plant biomass was positively correlated, explaining 16% of the variation in 2003 (P = 0.009) and 19% in 2004 (P = 0.002).
Proportion of leaves infected by (a) P. lagenophorae and (b) A. tragopogonis in 2003 and 2004 (N = 8). Infection varied significantly across plant species for both P. lagenophorae (2003: F 4, 27 = 88.23, P < 0.001; 2004: F 4, 27 = 5.54, P = 0.002) and A. tragopogonis: (2003: F 4, 27 = 185.20, P < 0.001; 2004: F 4, 27 = 74.79, P < 0.001). Dashes indicate no infection; different letters show significant differences among species in 2003 (a–d) and 2004 (w–z)
The fungicide treatment in 2004 reduced the abundance of P. lagenophorae (F 1, 27 = 8.22, P = 0.008) and A. tragopogonis (F 1, 27 = 10.33, P = 0.003), in each case by ~20%. Fungicide in 2003 was limited to soil and did not affect the abundance of leaf pathogens (P > 0.453). There was also no interaction of species and fungicide in either year (P > 0.181). The magnitude of pathogen reduction by fungicide ranged from 18–66% for P. lagenophorae and 18 to 86% for A. tragopogonis.
Belowground interactions of fungi with roots varied significantly across species for both root colonization by AM fungal hyphae (F 4, 10 = 12.14, P < 0.001) and root pathogens (F 4, 10 = 3.76, P = 0.041). Where AM fungi were most abundant, root pathogens were least abundant (r = −0.54, P = 0.037; Fig. 5). Root colonization by AM fungi increased by 37–59% and colonization by root pathogens decreased by 40–56% in the two exotics compared to the native and returned S. vulgaris, with intermediate levels of colonization in the hybrid (Fig. 5). Soil nematodes did not differ across species (P = 0.656).
Discussion
Origin, local experience, and biotic interactions
Origin was more important than local experience in the degree of biotic interactions we observed, despite large differences in the duration of post-introduction experience in the local range. The two exotic Senecio were generally subject to low infection by single pathogen species and suffered only low aphid infestation. In contrast, the native, returned, and hybrid Senecio experienced simultaneous infection by at least two pathogens plus infestation by aphids. Belowground, the exotics also had fewer root pathogens and more AM fungi compared to natives, with an inverse relationship suggesting that colonization by one may preclude the presence of the other (e.g., Borowicz 2001). The substantial reduction in negative interactions observed for the exotics regardless of local experience provides support for a mechanism underlying the enemy release hypothesis, that invasive species in a given habitat are more successful because they experience less damage relative to ecologically similar natives (Agrawal et al. 2005). There has been speculation as to whether mycorrhizal fungi may play a role in regulating invasiveness: the possibility revealed here that reduced impact of pathogenic soil fungi might increase the ability of the exotics to benefit form mutualistic mycorrhizal fungi is novel and may play a role in facilitating invasions.
Escape was not always the case, however, as the fungal agent of stem rot caused as much mortality in the exotics as in the natives. Nevertheless, the advantage provided by release from multiple enemies is one likely reason behind successful exotic establishment and spread; studies of other invasive Senecio species have shown that population growth rates decline and populations are more likely to go extinct when multiple enemies are present (McEvoy and Coombs 1999). The balance of multiple simultaneous interactions may better explain the success or failure of invasions than any single factor hypothesis (Mitchell et al. 2006).
Annual differences in the relative abundance of aphids and pathogens were substantial in some cases. For example, levels of P. lagenophorae and aphids increased dramatically on S. squalidus in 2004 and did not differ from the native Senecio species, while negative interactions remained negligible on the naïve S. ciliocarpa. Such year-to-year variation in enemy release may have been mediated by climate (e.g., Mueller and Buck 2003; e.g., Sullivan et al. 2002), with precipitation in April 2004 three times greater than in April 2003 (Hadley Centre 2006). Interactions of aphids and fungi may also play a role in their annual abundances, with multiple stresses increasing plant susceptibility, altering plant chemistry, and providing opportunities for establishment (e.g., Johnson et al. 2003). Though the specific driving forces behind pathogen and aphid infections are likely to be different given the small observed correlations in this study, annual variation in the degree of escape from enemies may reflect the ongoing development of local interactions.
Biotic interactions and plant fitness
The impact of biotic interactions on plant fitness is more informative than measures of the presence or abundance of mutualists and antagonists. Fitness consequences of rust pathogens, for example, can be very low despite incidence levels of 50–100% or be very high when incidence is less than 10% (see review in Roy and Kirchner 2000). Reducing pathogens with fungicide in this experiment didn’t change aboveground plant size, but did increase reproductive output by 25% primarily in the native, returned, and hybrid species. Increased reproductive output with fungicide application implies that the negative impact of the pathogenic fungi was greater than the positive impact of mycorrhizal associations, which would also have been reduced. In other studies, S. vulgaris infection by P. lagenophorae alone reduced reproductive output by 60% (Paul and Ayres 1986, 1987). Understanding the effects of interactions on plant fitness, rather than potential proxies such as biomass (which explained only 19% of the variation in plant reproduction in this study) or herbivore damage, allows for direct measurement of the importance of interactions to exotic plant success. Coupled with demographic models of population growth or rate of spread, we can begin to estimate the relative contributions of multiple factors to invasion success (e.g., Mitchell et al. 2006).
Plant fitness in terms of reproductive output was actually higher in the native species compared to the exotics in this study, regardless of local experience. Partly this was due to differences in plant phenology that were captured in the experiment. The native and returned S. vulgaris rapidly grow, set seed, and die. Both exotics, in contrast, were longer-lived, began flowering later, and flowered longer. In the case of S. ciliocarpa, no flowering occurred prior to the harvest date, but we observed flowering in August by plants that had not been harvested. Other studies of congeneric native and exotic species have found similar results, with generally greater reproduction in the native congeners (reviewed in Hawkes 2007). While clearly invaders must reproduce to be successful, they don’t necessarily need to reproduce more than native congeners in the same habitat. In the current comparison of Senecio species, differences in phenology and biotic interactions may be more important if they promote establishment of exotics and reduce competition among native and exotic congeners.
Origin vs. genetics
Interactions observed for the hybrid S. vulgaris × S. squalidus provided a test of the importance of genetics compared to origin, but the answer was dependent on the variable in question. In cases where the native S. vulgaris differed from the exotic S. squalidus, the hybrid ranged from intermediate between them (AMF root colonization 2003, root pathogen colonization 2003) to more like the native (aphid infestation 2003, Puccinia infection 2003, Albugo infection 2004) to more like the exotic (Puccinia infection 2004). There was also one case where the pathogen attack rate in the hybrid was greater than either the native or the exotic (Albugo infection 2003). The wide variety of results suggests that both genetics and experience are likely to matter, but further work will be required to understand their relative importance for any given trait.
Common garden plant species
Inference beyond this study may be limited by the lack of species replication within categories of experience. Individual species characteristics and life history differences were confounded with degree of experience, despite our attempt to minimize species variation using congeners. The use of a horticultural species raises some additional potential problems. Many horticultural species are bred for characteristics such as increased size, greater reproduction, or pest resistance (e.g., Crawley et al. 1996). If S. ciliocarpa was selected for resistance to disease or herbivory, this would potentially magnify enemy release compared to non-horticultural naïve species. To understand whether the patterns we observed apply more broadly, additional studies will be required.
Conclusions
In this group of Senecio species, interactions of exotic plants with the local biota appeared to be largely based on origin rather than the duration of local experience, though more variability was present in the historically introduced S. squalidus compared to the naïve S. ciliocarpa. The differences observed among species in this study are, of course, partly a consequence of their individual life histories. There was minimal evidence for the complete loss of enemy release or size advantages with increased experience—most patterns split by native versus exotic congeners. Observed release from pathogen and herbivore attack for invasive species is likely a reflection of the complex underlying landscape of multiple evolving biotic interactions among individual populations in the local habitat. Predicting how introduced plants will respond to and alter coevolutionary processes in the context of multiple, simultaneous interactions may be key to understanding the potential for initial and long-term invasiveness.
References
Agrawal AA, Kotanen PM, Mitchell CE, Power AG, Godsoe W, Klironomos J (2005) Enemy release? An experiment with congeneric plant pairs and diverse above- and belowground enemies. Ecology 86:2979–2989. doi:10.1890/05-0219
Benkman CW, Siepielski AM, Parchman TL (2008) The local introduction of strongly interacting species and the loss of geographic variation in species and species interactions. Mol Ecol 17:395–404. doi:10.1111/j.1365-294X.2007.03368.x
Berenbaum MR, Zangerl AR (2006) Parsnip webworms and host plants at home and abroad: trophic complexity in a geographic mosaic. Ecology 87:3070–3081. doi:10.1890/0012-9658(2006)87[3070:PWAHPA]2.0.CO;2
Blossey B, Notzold R (1995) Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. J Ecol 83:887–889. doi:10.2307/2261425
Borowicz VA (2001) Do arbuscular mycorrhizal fungi alter plant-pathogen relations? Ecology 82:3057–3068
Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D (2005) Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 144:1–11. doi:10.1007/s00442-005-0070-z
Burdon JJ, Thrall PH (1999) Spatial and temporal patterns in coevolving plant and pathogen associations. Am Nat 153:S15–S33. doi:10.1086/303209
CalFlora (2003) Information on California plants for education, research, and conservation [web application]. Retrieved from http://www.calflora.org/on Nov 30 2006 2006
Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443
Callaway RM, Ridenour WM, Laboski T, Weir T, Vivanco JM (2005) Natural selection for resistance to the allelopathic effects of invasive plants. J Ecol 93:576–583. doi:10.1111/j.1365-2745.2005.00994.x
Carroll S (2007) Brave new world: the epistatic foundations of natives adapting to invaders. Genetica 129:193–204. doi:10.1007/s10709-006-9014-8
Comes HP, Abbott RJ (2001) Molecular phylogeography, reticulation, and lineage sorting in Mediterranean Senecio sect. Senecio (Asteraceae). Evol Int J Org Evol 55:1943–1962
Crawley MJ, Harvey PH, Purvis A (1996) Comparative ecology of the native and alien floras of the British Isles. Philos Trans R Soc B Biol Sci 351:1251–1259. doi:10.1098/rstb.1996.0108
Ellstrand NC, Schierenbeck KA (2000) Hybridization as a stimulus for the evolution of invasiveness in plants? Proc Natl Acad Sci USA 97:7043–7050. doi:10.1073/pnas.97.13.7043
Elton CG (1958) The ecology of invasions by animals and plants. University of Chicago Press, Chicago, p 181
Fitter AH, Nichols R (1988) The use of benomyl to control infection by vesicular-arbuscular mycorrhizal fungi. New Phytol 110:201–206. doi:10.1111/j.1469-8137.1988.tb00253.x
Harris SA (2002) Introduction of Oxford ragwort, Senecio squalidus L. (Asteraceae), to the United Kingdom. Watsonia 24:31–43
Hadley Centre (2006) England and Wales Precipitation (HadEWP) 1766-present. Retrieved from http://www.metoffice.gov.uk/research/hadleycentre/obsdata/HadEWP.html on Nov 28 2006
Hawkes CV (2007) Are invaders moving targets? The generality and persistence of advantages in size, reproduction, and enemy release in invasive plant species with time since introduction. Am Nat 170:832–843. doi:10.1086/522842
James JK, Abbott RJ (2005) Recent, allopatric, homoploid hybrid speciation: The origin of Senecio squalidus (Asteraceae) in the British Isles from a hybrid zone on Mount Etna, Sicily. Evol Int J Org Evol 59:2533–2547
Johnson SN, Douglas AE, Woodward S, Hartley SE (2003) Microbial impacts on plant-herbivore interactions: the indirect effects of a birch pathogen on a birch aphid. Oecologia 134:388–396
Kahiluoto H, Vestberg M (2000) Creation of a non-mycorrhizal control for a bioassay of AM effectiveness: 1 Comparison of methods. Mycorrhiza 9:241–258. doi:10.1007/PL00009989
Koske RE, Gemma JN (1989) A modified procedure for staining roots to detect VA mycorrhizas. Mycol Res 92:486–488
Kraaijeveld AR, Godfray HCJ (1999) Geographic patterns in the evolution of resistance and virulence in Drosophila and its parasitoids. Am Nat 153:S61–S74. doi:10.1086/303212
Lowe AJ, Abbott RJ (2003) A new British species, Senecio eboracensis (Asteraceae), another hybrid derivative of S. vulgaris L. and S. squalidus L. Watsonia 24:375–388
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710. doi:10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2
McEvoy PB, Coombs EM (1999) Biological control of plant invaders: regional patterns, field experiments, and structured population models. Ecol Appl 9:387–401. doi:10.1890/1051-0761(1999)009[0387:BCOPIR]2.0.CO;2
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytol 115:495–501. doi:10.1111/j.1469-8137.1990.tb00476.x
Mitchell CE, Agrawal AA, Bever JD, Gilbert GS, Hufbauer RA, Klironomos JN, Maron JL, Morris WF, Parker IM, Power AG, Seabloom EW, Torchin ME, Vazquez DP (2006) Biotic interactions and plant invasions. Ecol Lett 9:726–740. doi:10.1111/j.1461-0248.2006.00908.x
Mueller DS, Buck JW (2003) Effects of light, temperature, and leaf wetness duration on daylily rust. Plant Dis 87:442–445. doi:10.1094/PDIS.2003.87.4.442
Newsham KK, Watkinson AR, West HM, Fitter AH (1995) Symbiotic fungi determine plant community structure–changes in a lichen-rich community induced by fungicide application. Funct Ecol 9:442–447. doi:10.2307/2390007
Paul ND, Ayres PG (1986) The impact of a pathogen (Puccinia lagenophorae) on populations of groundsel (Senecio vulgaris) overwintering in the field: II reproduction. J Ecol 74:1085–1094. doi:10.2307/2260235
Paul ND, Ayres PG (1987) Survival, growth and reproduction of groundsel (Senecio vulgaris) infected by rust (Puccinia lagenophorae) in the field during summer. J Ecol 75:61–71. doi:10.2307/2260536
Pelser PB, Gravendeel B, van der Meijden R (2002) Tackling speciose genera: species composition and phylogenetic position of Senecio sect: Jacobaea (Asteraceae) based on plastid and nrDNA sequences. Am J Bot 89:929–939. doi:10.3732/ajb.89.6.929
Pimentel D, Lach L, Zuniga R, Morrison D (2000) Environmental and economic costs of nonindigenous species in the United States. Bioscience 50:53–65. doi:10.1641/0006-3568(2000)050[0053:EAECON]2.3.CO;2
Pimentel D, McNair S, Janecka J, Wightman J, Simmonds C, O’Connell C, Wong E, Russel L, Zern J, Aquino T, Tsomondo T (2001) Economic and environmental threats of alien plant, animal, and microbe invasions. Agric Ecosyst Environ 84:1–20. doi:10.1016/S0167-8809(00)00178-X
Rejmanek M, Richardson DM (1996) What attributes make some plant species more invasive? Ecology 77:1655–1661. doi:10.2307/2265768
Rejmanek M, Pysek P, Prach K, Wade M (1995) What makes a species invasive? Plant invasions: general aspects and special problems. SPB Academic Publishing, Amsterdam, pp 3–13
Richardson DM, Allsopp N, D’Antonio CM, Milton SJ, Rejmanek M (2000) Plant invasions–the role of mutualisms. Biol Rev Camb Philos Soc 75:65–93. doi:10.1017/S0006323199005435
Roy BA, Kirchner JW (2000) Evolutionary dynamics of pathogen resistance and tolerance. Evol Int J Org Evol 54:51–63
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332. doi:10.1146/annurev.ecolsys.32.081501.114037
Smith MD, Hartnett DC, Rice CW (2000) Effects of long-term fungicide applications on microbial properties in tallgrass prairie soil. Soil Biol Biochem 32:935–946. doi:10.1016/S0038-0717(99)00223-0
SPSS (2005) SPSS for Windows. SPSS, Inc, Chicago
Strauss SY, Lau JA, Carroll SP (2006) Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecol Lett 9:357–374. doi:10.1111/j.1461-0248.2005.00874.x
Strayer DL, Eviner VT, Jeschke JM, Pace ML (2006) Understanding the long-term effects of species invasions. Trends Ecol Evol 21:645–651. doi:10.1016/j.tree.2006.07.007
Sullivan MJ, Damicone JP, Payton ME (2002) The effects of temperature and wetness period on the development of spinach white rust. Plant Dis 86:753–758. doi:10.1094/PDIS.2002.86.7.753
Thompson JN (1999) Specific hypotheses on the geographic mosaic of coevolution. Am Nat 153:S1–S14. doi:10.1086/303208
Thompson JN (2005) Coevolution: the geographic mosaic of coevolutionary arms races. Curr Biol 15:R992–R994. doi:10.1016/j.cub.2005.11.046
Thompson JN, Cunningham BM (2002) Geographic structure and dynamics of coevolutionary selection. Nature 417:735–738. doi:10.1038/nature00810
Whitehead AG, Hemming JR (1965) A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann Appl Biol 55:25–38. doi:10.1111/j.1744-7348.1965.tb07864.x
Acknowledgments
The authors would like to thank Dr. R. Raabe (UC Berkeley) and C. Abbott (U. York) for pathogen identification, Dr. D. Sivell (U. York) for non-aphid insect identifications, and Dr. L. Cole (CEH Banchory, UK) for nematode extractions. Seeds of S. ciliocarpa were kindly provided by B. Sims at Thompson and Morgan (UK) Ltd and those of S. vulgaris × S. squalidus by Dr. T. Crawford (U. York). We are also grateful to the following individuals who contributed to this project: A. Armstrong, O. Atkin, Z. Billings, E. Covey-Crump, T. Crawford, I. Hartley, A. Hodge, P. Ineson, C. Lancaster, M. Leibold, R. Raabe, J. Richards, P. Scott, D. Sherlock, M. Singer, and J. Zaragoza-Castells. Hawkes was supported by a National Science Foundation Postdoctoral Fellowship in Microbial Biology (DBI0200720).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hawkes, C.V., Douglas, A.E. & Fitter, A.H. Origin, local experience, and the impact of biotic interactions on native and introduced Senecio species. Biol Invasions 12, 113–124 (2010). https://doi.org/10.1007/s10530-009-9435-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-009-9435-2