Abstract
Three-dimensional cell culture technology is a novel cell culture technology, which can simulate the growth state of cells in vivo by scaffolds or special devices. Cells can form tissues or organs in vitro. It combines some advantages of traditional cell experiments and animal model experiments. Because of its advantages, it is widely used in clinical medical research, including research on stem cell differentiation, research on cell behavior, migration and invasion, study on microenvironment, study on drug sensitivity and radio-sensitivity of tumor cells, etc. In this paper, the evolution and classification of three-dimensional cell culture are reviewed, also the advantages and shortages are compared. The application of three-dimensional cell culture in clinical medicine are summarized to provide an insight into translational medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Three-dimensional (3D) cell culture is a novel cell culture method, which is applied to the researches of tumor cells and stem cells. The cells in vivo live in a microenvironment composed of adjacent tissue cells and the surrounding matrix. There are fibroblasts, immune cells, blood vessels and lymphatic vessels, and extracellular matrix in the surrounding matrix (Nazareth et al. 2007). Microenvironment is an important place for reaction, so its stability can ensure normal cell proliferation, differentiation, metabolism and functional activities (Behonick and Werb 2003). Cells can release cytokines into microenvironment through autocrine and paracrine to maintain survival conditions. Microenvironment, through the changes of metabolism, secretion, immunity, structure and function, conducts the changes of the environment in the whole body or in the distance, limiting and influencing the occurrence and development of regional cells (Mantovani et al. 2008). 3D culture technology is an in vitro culture method simulating the internal microenvironment. It constructs the external matrix skeleton and microenvironment of cell growth artificially, provides a culture condition similar to the internal environment, and enables cells to grow in a 3D way in vitro (Ovsianikov et al. 2012).
After the concept of three-dimensional cultivation technology was put forward, there were many related studies. One research direction is the selection and creation of scaffold materials. Finding a scaffold material with good biocompatibility and convenient preparation will be a breakthrough. In the field of tissue engineering, scaffold manufacturing technology based on electrospinning and 3D printing has achieved significant progress in replacing damaged tissues and organs at the laboratory level (Mabrouk et al. 2020). Related researches on nutrient dissemination in a three-dimensional culture environment have also attracted much attention. Nutrients (such as glucose, protein) needed by the cells in the scaffold to maintain growth. Different from the distribution of nutrients in conventional liquid media (Suhaimi et al. 2015a, b), in general, the diffusion coefficient of glucose increases with the increase of the pore size of the material (Suhaimi et al. 2015a, b). Mastering the diffusion characteristics can predict the distribution of nutrients in the cultured tissues, facilitating deeper studies of metabolism (Suhaimi and Das 2016).
The mechanical response, extracellular matrix, movement and migration, proliferation, differentiation, and gene expression of cells in the three-dimensional culture system and the two-dimensional system are different (Souza et al. 2018). The study of the differences in biological characteristics between the three-dimensional culture and the two-dimensional culture is also necessary. It is generally accepted that these differences are cell line specific (Duval et al. 2017). For example, liver cells and neuronal cells cannot survive well under traditional 2D culture methods (Ardalani et al. 2019; D'Aiuto et al. 2019). The ability of mesenchymal stem cells (MSCs) to develop into neurons, myoblasts and osteoblasts can be changed according to the different culture conditions of the matrix (Haugh et al. 2018). Before carrying out relevant three-dimensional research, it is often necessary to verify that the cell has not changed the characteristics related to the research purpose due to the culture environment. Researchers need to choose a suitable cell culture method according to the purpose of the experiment, rather than just believing that three-dimensional culture is better.
From the perspective of clinicians, there is no systematic review of the application of 3D cell culture in clinical medicine. This article focuses on the application of three-dimensional culture in stem cell differentiation-related research, tumor cell behavior research, migration and invasion, tumor microenvironment research, tumor cell drug sensitivity research, and tumor cell radiosensitivity research in recent years, and deepen researchers’ understanding of three-dimensional cells. This research can provide ideas for technology applications and translational medicine.
Advantages of three-dimensional culture over conventional technology
In the traditional two-dimensional culture technology, cells need to rely on the mechanical support of the bottom of the culture dish, then grow into monolayer cells attached to the bottom. Cells get even nutrition and oxygen in the surrounding environment, and finally achieve even and homogeneous growth (Duval et al. 2017). Under 3D culture conditions, cells show more real distribution and access to oxygen and nutrients, and the interaction between cells can also be reflected in the culture environment (Kapalczynska et al. 2018). Compared with the traditional two-dimensional culture technology, 3D culture technology can not only retain the shape of cells in vivo, but also reflect the intuitive and controllable conditions of cell culture, which is an ideal model for cell migration and differentiation. Compared with the partial polarization of cells in two-dimensional culture, it can provide a more accurate description of cell polarization (Baker and Chen 2012) (Fig. 1). The 3D environment allows cells to survive for up to 300 days and maintain healthy non-cancerous growth, which makes them more suitable for long-term research such as the long-term effects of drugs (Table 1). It is an experimental technology between animal experiments and two-dimensional cell culture.
Classification and evolution of three-dimensional culture technology
In 1968, Boiron invented and reported the 3D culture technology, and since then, the 3D culture technology began to develop rapidly (Boiron et al. 1968). The 3D culture can be divided into two kinds: the technique with scaffold and the technique without scaffold (Fig. 1). Cell scaffold technology can be divided into solid scaffolds and gel scaffolds according to the different scaffold materials. Moreover, scaffolds free can also achieve 3D cell culture. The principles are well explained by the schematic diagram (Fig. 2). According to the application of external forces, 3D cell culture types are divided into static 3D culture (Jianmin et al. 2001) and dynamic 3D culture (McKee et al. 2017; Yang et al. 2017).
Classification of three-dimensional cell culture techniques. The technology can be divided into three categories according to the scaffolds: gel scaffolds, solid scaffolds and scaffold free. The classic device pattern and cell morphology are shown in the figure above. a suspension drop culture plate. b low adhesion cell culture. c rotating bioreactor. d magnetic suspension. e Gel scaffolds. f solid scaffolds
Solid scaffolds
Solid materials use precise nanostructured materials as scaffolds, such as RGD peptide chain cross-linked sodium alginate (Dou et al. 2013), a scaffold network of carbon fibers to maintain cell growth (Liu et al. 2012). The cells grow between the complex nest-like scaffolds, which are also adherent to the wall in essence, but there are certain 3D structure and the interaction between cells. The preparation of scaffolds requires high requirements. It is usually necessary to use an electron microscope to detect the pore diameter of the scaffolds before the experiment, and only the scaffolds that meet the pore diameter can be tested in the next step. Whether the scaffold itself has an effect on cell survival also needs to be verified by experiments. Therefore, it is widely used in materials science and biomedical engineering.
Gel scaffolds
Gel scaffolds are a 3D skeleton composed of cross-linked polymers, including natural materials, such as chitin, collagen, hyaluronic acid and other ECM components, such as (Liu et al. 2016), polyester degradable polymers (Gümüşderelioğlu and Türkoğlu 2002). Polystyrene and polycaprolactone which can generate scaffolds similar to bone matrix structure are very popular in bone regeneration research (Patel et al. 2019). Chitin hydrogel has high cell compatibility, but poor mechanical properties. Chitin hydrogel infused into poly ε-caprolactone (PCL)/ nano-hydroxyapatite (nHA) scaffold with good mechanical properties. Results showed that it effectively promotes vascularization and osteoporosis, and it would be a promising application for bone regeneration. The collagen gel and glycosaminoglycan from animal origin have good biocompatibility, so they are also commonly used materials (Murray and Spector 2001). Special molecules and factors can also be added to the hydrogel according to specific experimental needs. Researchers achieved the substantial expansion of hematopoietic stem and progenitor cells in the degradable zwitterionic hydrogel which induced an inhibition of excessive reactive oxygen species (ROS) production via suppression of O + 2-related metabolism (Bai et al. 2019). The preparation procedure for gel scaffolds is relatively simple. It was easy to realize standardization by paying attention to the control of pH value and the temperature. However, gel scaffolds are usually not transparent enough to perform real-time imaging, but rather need to separate cells to successfully imaging (Zanoni et al. 2016). Hydrogels are not conducive to drug delivery in the study of hydrophobic drugs (Hoare and Kohane 2008). Therefore, the hydrogel is not a universal solution. Researchers need to decide on the skeleton properties according to specific experimental purposes.
Scaffold free
The principle of scaffold free culture is to prevent tumor cells from adhering to the wall by various physical methods, to suspend tumor cells in the culture medium, and to promote cell aggregation and growth to form tumor cell spheres. The culture methods include low adhesion cell culture plate (Eiraku and Sasai 2011), suspension drop culture plate (Weeks et al. 2013), rotating bioreactor (Samuelson and Gerber 2013), magnetic suspension (Haisler et al. 2013) and 3D printing (Zhao et al. 2014). On the low adhesion culture plate, the cells could not adhere to the wall, some cells died correspondingly, while the cells without death initially formed a round sphere. Then cells gradually form smaller spheres, which can screen out more aggressive tumor cell subcategories (Cordes et al. 2017). The culture medium in the suspension culture plate presents the shape of water drop and has no support, rather than the cylindrical shape on the traditional culture plate, so that the cells cannot be subjected to other forces except gravity, and finally realize the spherical growth, in the form of 96 well plate, it is convenient to realize the drug screening. Magnetic suspension is characterized by the presence of magnetic iron oxide and other substances in the culture medium, so that the external magnetic field can act on the whole culture medium to achieve suspension. The advantage of scaffold free culture is that cell detection is more convenient, most cells do not need to be separated from the medium, and can be directly used for the next step of experiments.
Static three-dimensional culture and dynamic three-dimensional culture
According to the application of external forces, 3D cell culture types are divided into static 3D culture (Jianmin et al. 2001) and dynamic 3D culture (McKee et al. 2017; Yang et al. 2017). Gravity is the only mechanical influence factor in the static 3D culture process. In order to achieve spherical growth in static culture, a special medium will be used, which contains no serum but high concentration of growth factors. At the same time, use a low-adhesion substance, such as 1.5% agar, to coat the culture dish to prevent cells from adhering to the surface to promote the formation of spherical cells (Derakhti et al. 2019). However, the stiffness of the culture medium can be used as an influencing factor to interfere with cells in a static system (Xu et al. 2019). The softer hydrogel helps mesenchymal stem cells differentiate into osteogenic cells (Žigon-Branc et al. 2019). Stress that can affect cells can be added in the dynamic 3D culture according to the needs of research. At present, it mainly includes shear force, tension and pressure (Kanazawa et al. 2014). The same cell line grows differently under static and dynamic culture environment. Stem cells from human exfoliated deciduous teeth can achieve better differentiation into hepatocytes in a dynamic environment (Huang et al. 2020). Researchers can choose different experimental method according to the specific purpose.
Application of three-dimensional culture
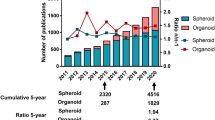
The clinical application of 3D culture can be divided into four categories: cell behavior, microenvironment, drug sensitivity, radiation sensitivity, and stem cell differentiation. Search for relevant keywords on pubmed, and the number of studies obtained (Table 2 and Fig. 3). Except for radiation sensitivity, hundreds of articles are published every year, which shows the popularity of related research.
The histogram of three-dimensional cell culture clinical studies published on Pubmed in the past five years. The number of researches on stem cells is the largest, followed by research on cell behavior related to cell migration and tumor metastasis, which reached a peak in 2018 and has gradually declined since then
Research on cell behavior
In 3D culture, cells have the ability to move in all directions, which has a great advantage in the study of cell behavior. In particular, for the study of tumor invasion and metastasis, the growth mode of cell clusters in the 3D culture system is poly condensation, and the cells in the central area are lack of oxygen and sufficient supply of nutrients, which is similar to the environmental characteristics of the cells in the central area of solid tumors in vivo, and better reflects the biological characteristics of tumor cells (Lewis et al. 2017). In 3D environment, the whole process of tumor formation can be recorded by a specific imaging system, which is conducive to long-term dynamic observation and tracking of tumor invasion and metastasis. Morales et al. (Morales and Alpaugh 2009) used in vitro 3D model to simulate the process of inflammatory breast cancer metastasis in vivo. The results showed that the effect of the collagen matrix on cell morphology and intercellular signal transduction in the 3D culture model was similar to that in vivo microenvironment, which affected the migration behavior of tumor cells. The researchers studied the interactions between colorectal cancer cells, fibroblasts, and endothelial cells in a three-dimensional cell culture environment, and conducted in vivo validation experiments in a mouse model. The results showed that the combined injection of endothelial cells significantly inhibited the growth of the primary tumor, while the combined injection of fibroblasts promoted the growth of lung metastasis of the tumor and reversed the effects of chemotherapy drugs. The invasion and growth of cancer cells in vitro culture can predict in vivo effects(Sarah et al. 2018). Researchers cultivated cervical cancer cells in a three-dimensional cell culture environment to track extracellular vesicles, which can carry and transmit mRNA and miRNA to promote tumor growth. They found that the secretion of extracellular vesicles increased, which indicated that previous two-dimensional related studies may have underestimated the metastasis risk (Thippabhotla et al. 2019).
Research on microenvironment
3D culture technology is suitable for analyzing the effect of microenvironment on cells, revealing some phenomena that cannot be found under traditional experimental methods. Su et al. (Su et al. 2015) Found that in the hCG rich microenvironment pretreated, the number of vascular network structures formed by hCG receptor-positive ovarian cancer cell lines (OVCAR-3 cells) and the expression levels of CD31, VEGF and factor VIII significantly increased. Park et al. (Park et al. 2017) determined the effect of 3D culture on the maintenance of undifferentiated porcine spermatogonial stem cells by analyzing the formation and morphology of cell colonies, transcription and translation regulation of genes related to self-renewal. Stem cells have stronger self-renewal ability in the 3D culture microenvironment than in the 2D culture microenvironment. Zhang et al. (Zhang et al. 2014) cell revealed that contraction collagen hydrogel could promote the growth of cells from G0/G1 phase to S phase, and promote DNA synthesis and cell proliferation. To some extent, these studies indicate that some experimental results in traditional two-dimensional cell culture may have an essential deviation.
Research on drug sensitivity
Monolayer cultured cells in vitro cannot reflect the pathophysiological structure and state of tumor cells in vivo. Therefore, it is a trend to study the drug sensitivity, dose, and efficacy in 3D culture. Perche et al. (Perche and Torchilin 2012) evaluated the efficacy of chemotherapy drugs by constructing 3D globules of cancer cells. In the model, adriamycin was used as a single drug or in combination with other anti-tumor drugs. In the 3D structure of cancer cells, the drug permeability was limited to the outer cell layer, indicating that globules have higher drug resistance than monolayer cells. Jung et al. constructed a 3D lung cancer model and studied the effects of Cisplatin and etoposide in standard chemotherapy regimens, providing important information to guide therapeutic approaches (Jung et al. 2019). The microfluidic chip is a new technology involving many disciplines. It integrates the basic operation units such as sample preparation, reaction, separation and detection into a micro-scale chip, automatically completes the whole process of sample analysisfor high-throughput screening of tumor drugs (Sabhachandani et al. 2015).
Research on radiation sensitivity
The radio-sensitivity and DNA damage repair ability of two-dimensional growth cells with the same genetic background are different from that of 3D growth cells. The experiment of radio-sensitivity in the 3D environment can better simulate the effect of radiotherapy on the tumor in vivo. Chan et al. utilized a 3D model to elucidate the multifactorial nature of radiation sensitivity in the culture of non-small cell lung cancer cell line A549 (Chan et al. 2016)0.3D cell radiation experiment is more suitable for clinical application. Gomez et al. (Gomez-Roman et al. 2016) cultured glioblastoma based on polystyrene scaffold, the 3D model reliably predicted the clinical efficacy, with potential value in pre-clinical evaluation. Sowa et al. (Sowa et al. 2010) carried out low let ionizing radiation on human mammary epithelial tissue in 2D and 3D culture environment to observed the effect of radiation-induced cytotoxicity. It is found that 3D cell culture has a protective effect on cell survival after irradiation, but a long-term culture in the 3D environment can significantly reduce the cytotoxicity at a given dose.
Research on stem cell differentiation
Stem cell differentiation is a multi-factor regulatory process, and also a hot research direction. Mesenchymal stem cells (MSCs) are a kind of adult pluripotent stem cells with multi-directional differentiation potential, which are derived from mesoderm and neuroectoderm, and do not express hematopoiesis related markers (Wang et al. 2012). According to the source, it can be divided into bone marrow mesenchymal stem cells, umbilical cord mesenchymal stem cells, synovial mesenchymal stem cells, and so on. The differentiation rate of MSCs in 2D culture environment was low. However, 3D culture technology can simulate the physiological environment of cells in the body, which is conducive to gene expression and signal transduction. Chaudhuri et al. (Chaudhuri et al. 2016) regulate the stress relaxation properties of hydrogels. It is found that different elasticity will affect the direction of differentiation of mesenchymal stem cells. Different elasticity can make stem cells differentiate into adipocytes, differentiate into osteoblasts, or do not differentiate. Goulart et al. used 3D bioprinting technology combined with autologous induced pluripotent stem cell (iPS)-derived transplantation technology to explore the treatment of patients with advanced liver disease and revealed the epithelial-mesenchymal transition resulting in rapid loss of hepatocyte phenotype in liver cancer (Goulart et al. 2019). The application of three-dimensional cell culture technology in the above fields has been summarized in the table for quick reading (Table 3).
Conclusion
3D cell culture has created a new experimental system and experimental design ideas. It can well simulate the internal environment, making the experimental results more convincing, and has been widely used in many frontier research fields. Compared with traditional experimental methods, 3D cell culture has many advantages, but there are still differences with the real human body, which provides the required skeleton for cell 3D growth, the simulation of related growth factors and the composition of tissue fluid in vivo environment is not enough, how to improve the culture conditions to simulate the conditions in vivo as much as possible is a direction for further research. And the price of 3D culture is relatively expensive, the process of standardization is not perfect, so there is some resistance in its promotion. With the progress and improvement of technology, 3D cell culture will become a fully used technology.
References
Ardalani H, Sengupta S, Harms V, Vickerman V, Thomson JA, Murphy WL (2019) 3-D culture and endothelial cells improve maturity of human pluripotent stem cell-derived hepatocytes. Acta Biomater 95:371–381. https://doi.org/10.1016/j.actbio.2019.07.047
Bai T, Li J, Sinclair A, Imren S, Merriam F, Sun F, O'Kelly MB, Nourigat C, Jain P, Delrow JJ, Basom RS, Hung HC, Zhang P, Li B, Heimfeld S, Jiang S, Delaney C (2019) Expansion of primitive human hematopoietic stem cells by culture in a zwitterionic hydrogel. Nat Med 25:1566–1575. https://doi.org/10.1038/s41591-019-0601-5
Baker BM, Chen CS (2012) Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci 125:3015–3024. https://doi.org/10.1242/jcs.079509
Behonick DJ, Werb Z (2003) A bit of give and take: the relationship between the extracellular matrix and the developing chondrocyte. Mech Dev 120:1327–1336. https://doi.org/10.1016/j.mod.2003.05.002
Boiron M, Guillemain B, Bernard C, Peries J, Chuat JC (1968) Presence in murine sarcoma virus stocks of a 3d component which alone initiates cellular conversion. Nature 219:748–749
Cattin S, Ramont L, Rüegg C (2018) Characterization and in vivo validation of a three-dimensional multi-cellular culture model to study heterotypic interactions in colorectal cancer cell growth invasion and metastasis. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2018.00097
Chan R, Sethi P, Jyoti A, McGarry R, Upreti M (2016) Investigating the radioresistant properties of lung cancer stem cells in the context of the tumor microenvironment. Radiat Res 185:169–181. https://doi.org/10.1667/RR14285.1
Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, Mooney DJ (2016) Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater 15:326–334. https://doi.org/10.1038/nmat4489
Cordes N, Nakano T, Kanai Y, Amano Y, Yoshimoto T, Matsubara D, Shibano T, Tamura T, Oguni S, Katashiba S, Ito T, Murakami Y, Fukayama M, Murakami T, Endo S, Niki T (2017) Establishment of highly metastatic KRAS mutant lung cancer cell sublines in long-term three-dimensional low attachment cultures. PLoS ONE 12:e0181342. https://doi.org/10.1371/journal.pone.0181342
D'Aiuto L, Bloom DC, Naciri JN, Smith A, Edwards TG, McClain L, Callio JA, Jessup M, Wood J, Chowdari K, Demers M, Abrahamson EE, Ikonomovic MD, Viggiano L, De Zio R, Watkins S, Kinchington PR, Nimgaonkar VL (2019) Modeling herpes simplex virus 1 infections in human central nervous system neuronal cells using two- and three-dimensional cultures derived from induced pluripotent stem cells. J Virol 93:e00111–e119. https://doi.org/10.1128/jvi.00111-19
Derakhti S, Safiabadi-Tali SH, Amoabediny G, Sheikhpour M (2019) Attachment and detachment strategies in microcarrier-based cell culture technology: a comprehensive review. Mater Sci Eng C Mater Biol Appl 103:109782. https://doi.org/10.1016/j.msec.2019.109782
Dou XQ, Li P, Zhang D, Feng CL (2013) RGD anchored C(2)-benzene based PEG-like hydrogels as scaffolds for two and three dimensional cell cultures. J Mater Chem B 1:3562–3568. https://doi.org/10.1039/c3tb20155d
Duval K, Grover H, Han LH, Mou Y, Pegoraro AF, Fredberg J, Chen Z (2017) Modeling physiological events in 2D vs. 3D cell culture. Physiology (Bethesda) 32:266–277. https://doi.org/10.1152/physiol.00036.2016
Eiraku M, Sasai Y (2011) Mouse embryonic stem cell culture for generation of three-dimensional retinal and cortical tissues. Nat Protoc 7:69–79. https://doi.org/10.1038/nprot.2011.429
Gomez-Roman N, Stevenson K, Gilmour L, Hamilton G, Chalmers AJ (2016) A novel 3D human glioblastoma cell culture system for modeling drug and radiation responses. Neuro-Oncol. https://doi.org/10.1093/neuonc/now164
Goulart E, de Caires-Junior LC, Telles-Silva KA, Araujo BHS, Rocco SA, Sforca M, de Sousa IL, Kobayashi GS, Musso CM, Assoni AF, Oliveira D, Caldini E, Raia S, Lelkes PI, Zatz M (2019) 3D bioprinting of liver spheroids derived from human induced pluripotent stem cells sustain liver function and viability in vitro. Biofabrication 12:015010. https://doi.org/10.1088/1758-5090/ab4a30
Gümüşderelioğlu M, Türkoğlu H (2002) Biomodification of non-woven polyester fabrics by insulin and RGD for use in serum-free cultivation of tissue cells. Biomaterials 23:3927–3935. https://doi.org/10.1016/s0142-9612(02)00128-x
Haisler WL, Timm DM, Gage JA, Tseng H, Killian TC, Souza GR (2013) Three-dimensional cell culturing by magnetic levitation. Nat Protoc 8:1940–1949. https://doi.org/10.1038/nprot.2013.125
Haugh MG, Vaughan TJ, Madl CM, Raftery RM, McNamara LM, O'Brien FJ, Heilshorn SC (2018) Investigating the interplay between substrate stiffness and ligand chemistry in directing mesenchymal stem cell differentiation within 3D macro-porous substrates. Biomaterials 171:23–33. https://doi.org/10.1016/j.biomaterials.2018.04.026
Hoare TR, Kohane DS (2008) Hydrogels in drug delivery: progress and challenges. Polymer 49:1993–2007. https://doi.org/10.1016/j.polymer.2008.01.027
Huang TY, Wang GS, Ko CS, Chen XW, Su WT (2020) A study of the differentiation of stem cells from human exfoliated deciduous teeth on 3D silk fibroin scaffolds using static and dynamic culture paradigms. Mater Sci Eng C Mater Biol Appl 109:110563. https://doi.org/10.1016/j.msec.2019.110563
Jianmin Z, Hongfang W, Meifu F (2001) Static three-dimensional culture of human hepatocarcinoma cell with microcarriers. Chin Sci Bull 46:1704–1708. https://doi.org/10.1007/bf02900656
Jung DJ, Shin TH, Kim M, Sung CO, Jang SJ, Jeong GS (2019) A one-stop microfluidic-based lung cancer organoid culture platform for testing drug sensitivity. Lab Chip 19:2854–2865. https://doi.org/10.1039/c9lc00496c
Kanazawa T, Nakagami G, Minematsu T, Yamane T, Huang L, Mugita Y, Noguchi H, Mori T, Sanada H (2014) Biological responses of three-dimensional cultured fibroblasts by sustained compressive loading include apoptosis and survival activity. PLoS ONE 9:e104676. https://doi.org/10.1371/journal.pone.0104676
Kapalczynska M, Kolenda T, Przybyla W, Zajaczkowska M, Teresiak A, Filas V, Ibbs M, Blizniak R, Luczewski L, Lamperska K (2018) 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch Med Sci 14:910–919. https://doi.org/10.5114/aoms.2016.63743
Lewis DM, Blatchley MR, Park KM, Gerecht S (2017) O(2)-controllable hydrogels for studying cellular responses to hypoxic gradients in three dimensions in vitro and in vivo. Nat Protoc 12:1620–1638. https://doi.org/10.1038/nprot.2017.059
Liu H, Liu J, Qi C, Fang Y, Zhang L, Zhuo R, Jiang X (2016) Thermosensitive injectable in-situ forming carboxymethyl chitin hydrogel for three-dimensional cell culture. Acta Biomater 35:228–237. https://doi.org/10.1016/j.actbio.2016.02.028
Liu Y, Li X, Qu X, Zhu L, He J, Zhao Q, Wu W, Li D (2012) The fabrication and cell culture of three-dimensional rolled scaffolds with complex micro-architectures. Biofabrication 4:015004. https://doi.org/10.1088/1758-5082/4/1/015004
Mabrouk M, Beherei HH, Das DB (2020) Recent progress in the fabrication techniques of 3D scaffolds for tissue engineering. Mater Sci Eng C 110:110716. https://doi.org/10.1016/j.msec.2020.110716
Mantovani A, Pedro Romero A, Palucka K, Marincola FM (2008) Tumour immunity: effector response to tumour and role of the microenvironment. Lancet 371:771–783. https://doi.org/10.1016/s0140-6736(08)60241-x
McKee C, Hong Y, Yao D, Chaudhry GR (2017) Compression induced chondrogenic differentiation of embryonic stem cells in three-dimensional polydimethylsiloxane scaffolds. Tissue Eng Part A 23:426–435. https://doi.org/10.1089/ten.TEA.2016.0376
Morales J, Alpaugh ML (2009) Gain in cellular organization of inflammatory breast cancer: A 3D in vitro model that mimics the in vivo metastasis. BMC Cancer 9:462. https://doi.org/10.1186/1471-2407-9-462
Murray MM, Spector M (2001) The migration of cells from the ruptured human anterior cruciate ligament into collagen-glycosaminoglycan regeneration templates in vitro. Biomaterials. https://doi.org/10.1016/s0142-9612(00)00426-9
Nazareth MR, Broderick L, Simpson-Abelson MR, Kelleher RJ, Yokota SJ, Bankert RB (2007) Response to comment on “characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells”. J Immunol 179:733–833. https://doi.org/10.4049/jimmunol.179.2.733
Ovsianikov A, Mironov V, Stampf J, Liska R (2012) Engineering 3D cell-culture matrices: multiphoton processing technologies for biological and tissue engineering applications. Expert Rev Med Devices 9:613–633. https://doi.org/10.1586/erd.12.48
Park JE, Park MH, Kim MS, Park YR, Yun JI, Cheong HT, Kim M, Choi JH, Lee E, Lee ST (2017) Porcine spermatogonial stem cells self-renew effectively in a three dimensional culture microenvironment. Cell Biol Int 41:1316–1324. https://doi.org/10.1002/cbin.10844
Patel BB, Sharifi F, Stroud DP, Montazami R, Hashemi NN, Sakaguchi DS (2019) 3D microfibrous scaffolds selectively promotes proliferation and glial differentiation of adult neural stem cells: a platform to tune cellular behavior in neural tissue engineering. Macromol Biosci. https://doi.org/10.1002/mabi.201800236
Perche F, Torchilin VP (2012) Cancer cell spheroids as a model to evaluate chemotherapy protocols. Cancer Biol Ther 13:1205–1213
Sabhachandani P, Motwani V, Cohen N, Sarkar S, Konry T (2015) Generation and functional assessment of 3D multicellular spheroids in droplet based microfluidics platform. Lab Chip 16:497–505
Samuelson L, Gerber DA (2013) Improved function and growth of pancreatic cells in a three-dimensional bioreactor environment. Tissue Eng Part C Methods 19:39–47. https://doi.org/10.1089/ten.TEC.2012.0236
Souza AG, Silva IBB, Campos-Fernandez E, Barcelos LS, Souza JB, Marangoni K, Goulart LR, Alonso-Goulart V (2018) Comparative assay of 2D and 3D cell culture models: proliferation, gene expression and anticancer drug response. Curr Pharm Des 24:1689–1694. https://doi.org/10.2174/1381612824666180404152304
Sowa MB, Chrisler WB, Zens KD, Ashjian EJ, Opresko LK (2010) Three-dimensional culture conditions lead to decreased radiation induced cytotoxicity in human mammary epithelial cells. Mutat Res 687:78–83. https://doi.org/10.1016/j.mrfmmm.2010.03.004
Su M, Fan C, Gao S, Shen A, Zhang Y (2015) An HCG-rich microenvironment contributes to ovarian cancer cell differentiation into endothelioid cells in a three-dimensional culture system. Oncol Rep 34:2395
Suhaimi H, Das DB (2016) Glucose diffusivity in cell-seeded tissue engineering scaffolds. Biotechnol Lett 38:183–190. https://doi.org/10.1007/s10529-015-1958-2
Suhaimi H, Wang S, Das DB (2015a) Glucose diffusivity in cell culture medium. Chem Eng J 269:323–327. https://doi.org/10.1016/j.cej.2015.01.130
Suhaimi H, Wang S, Thornton T, Das DB (2015b) On glucose diffusivity of tissue engineering membranes and scaffolds. Chem Eng Sci 126:244–256. https://doi.org/10.1016/j.ces.2014.12.029
Thippabhotla S, Zhong C, He M (2019) 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci Rep 9:13012. https://doi.org/10.1038/s41598-019-49671-3
Wang S, Qu X, Zhao RC (2012) Clinical applications of mesenchymal stem cells. J Hematol Oncol 5:19. https://doi.org/10.1186/1756-8722-5-19
Weeks CA, Newman K, Turner PA, Rodysill B, Hickey RD, Nyberg SL, Janorkar AV (2013) Suspension culture of hepatocyte-derived reporter cells in presence of albumin to form stable three-dimensional spheroids. Biotechnol Bioeng 110:2548–2555. https://doi.org/10.1002/bit.24899
Xu K, Ganapathy K, Andl T, Wang Z, Copland JA, Chakrabarti R, Florczyk SJ (2019) 3D porous chitosan-alginate scaffold stiffness promotes differential responses in prostate cancer cell lines. Biomaterials 217:119311. https://doi.org/10.1016/j.biomaterials.2019.119311
Yang L, Carrington LJ, Erdogan B, Ao M, Brewer BM, Webb DJ, Li D (2017) Biomechanics of cell reorientation in a three-dimensional matrix under compression. Exp Cell Res 350:253–266. https://doi.org/10.1016/j.yexcr.2016.12.002
Zanoni M, Piccinini F, Arienti C, Zamagni A, Santi S, Polico R, Bevilacqua A, Tesei A (2016) 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci Rep 6:19103. https://doi.org/10.1038/srep19103
Zhang X, Zhang Y, Chen W, Xu L, Wei S, Zheng Y, Zhai M (2014) Biological behavior of fibroblast on contractile collagen hydrogel crosslinked by gamma-irradiation. J Biomed Mater Res A 102:2669–2679. https://doi.org/10.1002/jbm.a.34938
Zhao Yu, Yao R, Ouyang L, Ding H, Zhang T, Zhang K, Cheng S, Sun W (2014) Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 6:035001. https://doi.org/10.1088/1758-5082/6/3/035001
Žigon-Branc S, Markovic M, Van Hoorick J, Van Vlierberghe S, Dubruel P, Zerobin E, Baudis S, Ovsianikov A (2019) Impact of hydrogel stiffness on differentiation of human adipose-derived stem cell microspheroids. Tissue Eng Part A 25:1369–1380. https://doi.org/10.1089/ten.TEA.2018.0237
Funding
Funding was provided by the National Natural Science Foundation of China (No. 81871538).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Q., Wang, Y. The application of three-dimensional cell culture in clinical medicine. Biotechnol Lett 42, 2071–2082 (2020). https://doi.org/10.1007/s10529-020-03003-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-03003-y