Abstract
Objectives
To realize a practical technology for recycling both cyclodextrin and resting-cells at the same time in phytosterol biotransformation using mycobacterial resting cells.
Results
In order to produce 22-hydroxy-23,24-bisnorchol-4-ene-3-one (HBC) efficiently and low-costly, a recycled phytosterols (PS) biotransformation process using mycobacterial resting cells was developed. By optimizing the ratio of hydroxypropyl-β-cyclodextrin (HP-β-CD) and PS to 1:1 (w/w), most products crystallized during the biotransformation process. So, the HBC was easily separated by low-speed (900×g) centrifugation with yield of 92%. The resting cells, HP-β-CD and the residual products and substrates left in the reaction system were reused for another bioconversion cycle after PS supplement. Three continuous cycles were achieved without the supplement of cells and HP-β-CD. In each batch, 80 g L−1 of PS was transformed to HBC with the space–time yield of HBC of 8.9–12.8 g L−1 day−1.
Conclusions
This strategy reduced the cost of HBC production and simplified the purification process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mycobacterium can degrade side-chain of cholesterol, phytosterols (PS) as carbon source to produce valuable steroidal intermediates (Donova et al. 2005), such as 22-hydroxy-23,24-bisnorchol-4-ene-3-one (HBC). It is one of the important steroidal intermediates that can be used to synthesize progesterone and adrenocortical hormone (Donova and Egorova 2012; Toró and Ambrus 1990). Xu et al. reported an engineered Mycobacterium neoaurum ATCC 25795 transformed PS to HBC efficiently (Xu et al. 2016).
Nevertheless, there are still some obstacles due to poor water-solubility (less than 0.1 mmol L−1) and bio-toxic of PS that hamper the biotransformation process (Stefanov et al. 2006). Many approaches have been taken to improve the transformation efficiency of PS, including cyclodextrin complexing technology, organic solvent solution-promoting, two phase water–oil systems or surfactants (Stefanov et al. 2006; Manosroi et al. 2008; Heipieper et al. 2007). Among these approaches, cyclodextrin complexing technology was testified an effective method to enhance the transformation of sterols (Fernandes et al. 2003; Hesselink et al. 1989). The conversion rate of PS can be increased by 2–3 times in the presence of cyclodextrin (Zhou et al. 2019; Shtratnikova et al. 2017). However, the high price of cyclodextrin limited its industrial application (Mancilla et al. 2018), so that the cyclic utilization of cyclodextrin was needed. In our previous works, HP-β-CD was recycled after the production extracted by ethyl acetate (Gao et al. 2015). Shen et al. immobilized cyclodextrin on loofah fiber for its cyclic utilization in biotransformation (Shen et al. 2017). However, these approaches to recycling HP-β-CD were cumbersome and costly, which was not conducive to industrial application. Moreover, it did not realize the recycle of cells in the same time.
The purpose of this study was to reuse the HP-β-CD and resting-cells at the same time in the phytosterol biotransformation and develop a recycled batch process for efficient and low-cost production of HBC with high concentration of PS.
Materials and methods
Strain, reagents and medium
An engineered Mycobacterium neoaurum WIII was constructed by Xu et al. (2016). It transformed PS into HBC as main product. PS (95.7% purity) were purchased from Shanxi Sciphar Natural Products Co., Ltd. (Shangluo, China). Industrial grade HP-β-CD was purchased from Shandong Binzhou Zhiyuan Biotechnology Co., Ltd. (Binzhou, China). Corn steep powder was purchased from Xiwang Group Co., Ltd. (Binzhou, China). AD, 1,4-HBC, HBC standards were obtained from Sigma (Shanghai, China). The methanol, acetonitrile and isopropanol used in the HPLC analysis were of chromatographic grade, while other chemical reagents were of analytical purity. MYC/01 was used as the seed medium and MYC/04 as the medium for the preparation of resting cells. MYC/01 (g L−1): K2HPO4·3H2O 0.5; MgSO4·7H2O 0.5; ammonium ferric citrate 0.05; sodium citrate 2.7; NH4Cl 2.7; glycerol 20; pH 8.0 (Gao et al. 2015). MYC/04 (g L−1): sodium citrate 2.7; glucose 40; (NH4)2SO4 1.0; (NH4)2HPO4 10; corn steep powder 3; β-CD 3.0; PS 0.1; pH 8.0.
Preparation of resting cells and PS biotransformation
The culture and harvesting of mycobacterial resting cells were carried out according to the method described by Gao et al. (2014). The PS biotransformation was performed in 20 mL phosphate buffer (20 mmol L−1, pH 8.0) under non-sterile condition in a 250-mL flask at 30 °C with a shaking speed of 200 rpm, containing the resting cell, PS and HP-β-CD according to the requirements. The cell density was determined by dry cell weight or the total cellular protein of cell lysate (Saab et al. 2013). All experiments were repeated three times separately.
Analysis of products and substrates
Samples (100 μL) were withdrawn at interval and extracted by ten times volume of ethyl acetate according to our previous work (Gao et al. 2014). Steroidal intermediates and PS were analyzed by high performance liquid chromatography (HPLC, Agilent Technologies, Inc., Santa Clara, CA, USA) equipped with Agilent ZORBAX SB-C18 column (5 μm, 4.6 × 250 mm). For the analysis of PS, acetonitrile and isopropanol (70:30, v/v) were used as the mobile phase at 1 mL min−1, and a UV detector at 210 nm was used. For the analysis of steroidal intermediates, methanol and water (80:20, v/v) were used as the mobile phase at 1 mL min−1, and a UV detector at 254 nm was used.
Results and discussion
Effect of the HP-β-CD and PS ratio in the reaction system on biotransformation
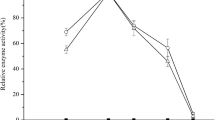
According to previous reports, the HP-β-CD could solubilize the PS in the transformation process at the condition of both growing cells and resting cells with the optimal mass ratio of HP-β-CD and PS was 4:1 (molar ratio, 1:1) (Gao et al. 2015; Jambhekar and Breen 2016). Considering the high price of HP-β-CD, the dosage of HP-β-CD was reduced to cater for its application in the industrial scale. As shown in Fig. 1, when the mass ratio of HP-β-CD and PS decreased from 4:1 to 1:1, the concentration of the HBC still remained at about 35.5 g L−1 after 5 days of transformation. When the mass ratio of HP-β-CD and PS decreased to 1:2, the production rate of HBC decreased significantly and the maximum concentration of the HBC was 33.5 g L−1. This result was different from previous reports which the final yield of product would reduce observably when the molar ratio of HP-β-CD and PS was lower than 1:1 (mass ratio was 4:1) (Gao et al. 2015). The reaction rate could remain at a higher level even the mass ratio of HP-β-CD and PS decreased to 1:1. But further decreasing the mass ratio of HP-β-CD and PS to 1:2, the solubilizing effect of HP-β-CD could be affected and the reaction rate decreased correspondently.
Profile of the PS biotransformation with different mass ratio of HP-β-CD. PS biotransformation was carried out in 250-mL flasks containing 50 g L−1 PS and different amount of HP-β-CD with the resting cell (9.0 g L−1 DCW) for 5 days. Data were the average value from triplicate experiments ± standard deviation
Further researches found the formation of the HBC tabular crystal during the biotransformation because of the low solubility and high concentration of HBC in the reaction system. Moreover, the less HP-β-CD was added, the more crystals were precipitated due to the decreased solubilization of HP-β-CD. By optimizing the ratio of HP-β-CD and PS to 1:1 (w/w), most products crystallized during the biotransformation process that could alleviate the product inhibition to cell metabolism (Wang et al. 2009). As a result, the cost of HP-β-CD decreased and, in the meantime, it will simplify the downstream processing because the crystal product is easy to separate from the reaction system.
Improvement of the space–time yield of HBC
In order to enhance the space–time yield of HBC, the concentration of resting cells and PS-HP-β-CD suspension (HP-β-CD:PS = 1:1, w/w) in the transformation system were optimized in 250-mL flasks. As shown in Table 1, the highest space–time yield of HBC reached 7.87 g L−1 day−1 when 12.0 g L−1 DCW and 80 g L−1 PS were applied in the reaction system. Further increasing the concentration of resting cells and PS decreased the space–time yield of HBC. This was because the high concentration of resting cells and PS gave rise to the high viscosity of the reaction system and high oxygen uptake rate. The oxygen availability became a main restricting factor for the biotransformation of PS determined by the oxygen transfer rate (Gao et al. 2014).
Recycled batch biotransformation in a 3.7 L fermenter
Because the HBC crystal could be easily separated from the reaction system, a recycled batch biotransformation was designed in a 3.7-L fermenter (Fig. 2). PS biotransformation was carried out in fermenter under the optimized condition abovementioned. The dissolved oxygen level in bioprocess was kept to more than 30% by adjusting airflow and agitation. In the end of biotransformation, the HBC was separated by low speed centrifugation (900×g), and the resting cells and HP-β-CD remained in the reaction system. The precipitation, mainly including HBC and a small quantity of resting cells, could be extracted by organic solvent to obtain HBC. The reaction system, by supplementing the PS, was directly reused in the next biotransformation process. This strategy could significantly simplify the bioprocess of HBC production by recycling usage of the reaction system and reduce the production cost. At the same time, this method was environmentally friendly because it reduced the wastewater discharge in the biotransformation process. It was extremely suitable for industrial application.
As shown in Table 2, the first batch biotransformation ended at the 4.5th day when the highest concentration of HBC (57.4 g L−1) was obtained. The space–time yield of HBC reached 12.8 g L−1 day−1 in fermenter, 1.6 times higher compared with that in shake flasks. It was due to the better mass transfer rate (including oxygen) in fermenter that resulted in a higher reaction rate (Gao et al. 2014). After the separation of HBC from the reaction system by centrifugation, the residue HBC was only 6.5 g L−1, no more than 8% of the total products and resting-cells accounted for 70% of the initial concentration. In the consecutive batch biotransformation, the reaction rate was gradually decreased. By the third batch, 92.4% of the substrate was consumed within 6 days and the space–time yield of HBC was only 69.5% of the first batch. This was mainly due to the loss of the resting cells in the process of HBC separation. Actually, the reaction rate of the following recycled batches could be improved by moderately supplementing the resting cells in each batch biotransformation. In addition, HP-β-CD is a stable chemical that could be recycled as a co-solvent many times after simple processing (Gao et al. 2015). That could further reduce the cost of the biotransformation process.
Conclusions
A recycled batch biotransformation process from high concentration of PS (80 g L−1) to HBC was developed. HP-β-CD and resting-cells were reused in this strategy and the space–time yield of HBC reached 8.9–12.8 g L−1 day−1 in three recycled batch biotransformation, which was the highest level reported. These results indicated that the recycled batch biotransformation was a feasible transformation strategy, which could observably shorten the reaction period, be beneficial to the separation and purification of HBC, reduce production cost and be environmentally friendly. It has great application prospects for production of valuable compounds from PS by microorganism in industry through precipitation strategy.
References
Donova MV, Egorova OV (2012) Microbial steroid transformations: current state and prospects. Appl Microbiol Biotechnol 94:1423–1447. https://doi.org/10.1007/s00253-012-4078-0
Donova MV, Dovbnya DV, Sukhodolskaya GV, Khomutov SM, Nikolayeva VM, Kwon I, Han K (2005) Microbial conversion of sterol-containing soybean oil production waste. J Chem Technol Biotechnol 80(1):55–60. https://doi.org/10.1002/jctb.1156
Fernandes P, Cruz A, Angelova B, Pinheiro HM, Cabral JMS (2003) Microbial conversion of steroid compounds: recent developments. Enzym Microb Technol 32:688–705. https://doi.org/10.1016/s0141-0229(03)00029-2
Gao XQ, Feng JX, Hua Q, Wei DZ, Wang XD (2014) Investigation of factors affecting biotransformation of phytosterols to 9-hydroxyandrost-4-ene-3,17-dione based on the HP-β-CD-resting cells reaction system. Biocatal Biotransform 32:343–347. https://doi.org/10.3109/10242422.2014.976633
Gao XQ, Feng JX, Wang XD, Hua Q, Wei DZ (2015) Enhanced steroid metabolites production by resting cell phytosterol bioconversion. Chem Biochem Eng Quart 29:567–573. https://doi.org/10.15255/CABEQ.2014.2098
Heipieper HJ, Neumann G, Cornelissen S, Meinhardt F (2007) Solvent-tolerant bacteria for biotransformations in two-phase fermentation systems. Appl Microbiol Biotechnol 74:961–973. https://doi.org/10.1007/s00253-006-0833-4
Hesselink P, Vliet S, Vries H, Witholt B (1989) Optimization of steroid side chain cleavage by Mycobacterium sp. in the presence of cyclodextrins. Enzym Microb Technol 11:398–404. https://doi.org/10.1016/0141-0229(89)90133-6
Jambhekar S, Breen P (2016) Cyclodextrins in pharmaceutical formulations II: solubilization, binding constant, and complexation efficiency. Drug Discov Today 21:363–368. https://doi.org/10.1016/j.drudis.2015.11.016
Manosroi A, Saowakhon S, Manosroi J (2008) Enhancement of androstadienedione production from progesterone by biotransformation using the hydroxypropyl-beta-cyclodextrin complexation technique. J Steroid Biochem Mol Biol 108:132–136. https://doi.org/10.1016/j.jsbmb.2007.05.032
Mancilla RA, Little C, Amoroso A (2018) Efficient bioconversion of high concentration phytosterol microdispersion to 4-androstene-3,17-dione (AD) by Mycobacterium sp. B3805. Appl Biochem Biotechnol 185:494–506. https://doi.org/10.1007/s12010-017-2665-3
Stefanov S, Yankov D, Beschkov V (2006) Biotransformation of phytosterols to androstenedione in two phase water-oil systems. Chem Biochem Eng Quart 20:421–427
Shtratnikova VY, Schelkunov MI, Dovbnya DV, Bragin EY, Donova MV (2017) Effect of methyl-β-cyclodextrin on gene expression in microbial conversion of phytosterol. Appl Microbiol Biotechnol 101:4659–4667. https://doi.org/10.1007/s00253-017-8288-3
Shen YB, Yu ZQ, Yang X, Wang F, Luo JM, Wang M (2017) A new technique for promoting cyclic utilization of cyclodextrins in biotransformation. J Ind Microbiol Biotechnol 44:1–7. https://doi.org/10.1007/s10295-016-1856-1
Saab HB, Fouchard S, Boulanger A, Llopiz P, Neunlist S (2013) Luffa cylindrica and phytosterols bioconversion: from shake flask to jar bioreactor. J Ind Microbiol Biotechnol 40:315–1320. https://doi.org/10.1007/s10295-013-1315-1
Toró A, Ambrus G (1990) Oxidative decarboxylation of 17(20)-dehydro-23,24-dinorcholanoic acids. Tetrahedron Lett 31:3475–3476. https://doi.org/10.1016/S0040-4039(00)97426-4
Wang M, Zhang L, Shen Y, Ma Y, Zheng Y, Luo J (2009) Effects of hydroxypropyl-β-cyclodextrin on steroids 1-en-dehydrogenation biotransformation by Arthrobacter simplex TCCC 11037. J Mol Catal B 59:58–63. https://doi.org/10.1016/j.molcatb.2008.12.017
Xu LQ, Liu YJ, Yao K, Liu HH, Tao XY, Wang FQ, Wei DZ (2016) Unraveling and engineering the production of 23,24-bisnorcholenic steroids in sterol metabolism. Sci Rep 6:21928. https://doi.org/10.1038/srep21928
Zhou X, Zhang Y, Shen YB, Zhang X, Xu SP, Shang ZH, Xia ML, Wang M (2019) Efficient production of androstenedione by repeated batch fermentation in waste cooking oil media through regulating NAD+/NADH ratio and strengthening cell vitality of Mycobacterium neoaurum. Bioresour Technol 279:209–217. https://doi.org/10.1016/j.biortech.2019.01.144
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Numbers 31570079, 21276083).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, Y., Wang, D., Wang, X. et al. A recycled batch biotransformation strategy for 22-hydroxy-23,24-bisnorchol-4-ene-3-one production from high concentration of phytosterols by mycobacterial resting cells. Biotechnol Lett 42, 2589–2594 (2020). https://doi.org/10.1007/s10529-020-02991-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-02991-1