Abstract
Objectives
The synergistic effects between cellulases and lytic polysaccharide monooxygenases (LPMOs) were investigated systematically in terms of their degree of synergy (DS) on amorphous and crystalline cellulose. Synergy curves were obtained for enzyme pairs containing a cellulase from Trichoderma reesei (Cel6A and Cel7A) and three LPMOs from Thermoascus aurantiacus (TaAA9A), Lentinus similis (LsAA9A) and Thielavia terrestris (TtAA9E).
Results
The synergistic experiments showed that the three LPMOs significantly improved the hydrolytic efficiency of Cel6A, on both cellulosic substrates; a more pronounced effect being seen for TtAA9E on amorphous cellulose at low cellulase:LPMO ratios. In contrast, the highly processive, reducing-end acting Cel7A synergised with the C1-C4 oxidising LPMOs, TaAA9A and LsAA9A, but was inhibited by the presence of C1-oxidizing TtAA9E.
Conclusions
The degree of synergy exhibited by the cellulase-LPMO mixtures was enzyme- and substrate-specific. The observed Cel7A inhibition, rather than synergy, by the C1-oxidizing LPMO, TtAA9E, warrants further investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overcoming the recalcitrance of the plant cell wall, and of cellulose in particular, is important for the more effective use of lignocellulosic biomass as an industrial feedstock. It has long been clear that cellulose is enzymatically hydrolysed by a variety of cellulases (β-1,4-glycoside hydrolases) that act in concert to produce glucose (Mandels and Reese 1965). This concerted effect is observed when the combination of two or more cellulases leads to a higher degree of glucosidic-bond cleavage than the sum of cleavages by each of the individual cellulases. In the conventional understanding of this synergistic action between cellulases, enzymes with endolytic character create new chain ends in the substrate, improving accessibility and increasing the number of attack sites for exolytic cellulases (Väljamäe et al. 1999; Boisset et al. 2001). Furthermore, synergy can also result from the underlying differences in the specificity of the enzymes towards particular regions of the cellulosic substrate (Jalak et al. 2012).
Subsequent to early work on cellulase synergy by Boisset et al. (Boisset et al. 2001), a family of oxidative enzymes has been shown to possess an important endo-acting cellulolytic activity. They are termed lytic polysaccharide monooxygenases (LPMOs). The synergistic action of LPMOs and cellulases is currently being utilized commercially to increase sugar yields in lignocellulose hydrolysis (Johansen 2016). It has been shown that lignocellulose conversion can be boosted by the addition of LPMOs (Harris et al. 2010; Eibinger et al. 2014; Hu et al. 2014; Müller et al. 2015). For example, cellulase-LPMO synergy has been reported for both exo- and endo-acting cellulases from Trichoderma reesei (Cel7A, Cel6A, Cel5A and Cel7B), with a more pronounced effect being seen for Cel7A (Quinlan et al. 2011).
However, few systematic studies, using several molar fractions of enzymes, have yet been carried out on the synergy between individual LPMOs and cellulases. This paper presents a detailed investigation of cellulase-LPMO synergy, using well-defined enzyme preparations. We studied the rate of product formation using different molar ratios of a cellulase and an LPMO (Cel6A and Cel7A from Trichoderma reesei with three LPMOs from Thermoascus aurantiacus (TaAA9A), Lentinus similis (LsAA9A) and Thielavia terrestris (TtAA9E)). We systematically investigated the synergy of the enzyme mixtures on amorphous and crystalline cellulose by using a wide range of enzymatic ratios.
Materials and methods
Materials and chemicals
Avicel PH-101, para-hydroxybenzoic acid hydrazide, potassium sodium tartrate tetrahydrate and gluconic acid were purchased from Sigma-Aldrich. Avicel was thoroughly washed with deionised water before the preparation of an Avicel stock solution in 50 mM Citrate/Phosphate buffer, pH 6. Phosphoric acid swollen cellulose (PASC) was prepared from Avicel as described previously (Kracher et al. 2018), followed by thorough washing with deionised water, before the preparation of a PASC stock solution in 50 mM Citrate/Phosphate buffer (pH 6). Earlier work has shown that Avicel has a crystallinity index of about 0.50 while PASC is almost entirely amorphous (Zhang and Lynd 2004).
Enzyme production and purification
The two cellulases from Trichoderma reesei, Cel6A and Cel7A, were heterologously expressed in Aspergillus oryzae and purified as described previously (Westh et al. 2014). We used three well-characterized fungal LPMOs, classified in the auxiliary activity family 9 (AA9) in the CAZy database: TaAA9A from Thermoascus aurantiacus (Harris et al. 2010) and LsAA9A from Lentinus similis (Frandsen et al. 2016), both employ a mixed C1/C4 oxidative mechanism, and TtAA9E (GH61E) from Thielavia terrestris (Harris et al. 2010) employs a C1-oxidative mechanism. The LPMOs were kindly provided by Novozymes A/S. The batch of TaAA9A was prepared as described previously (Scott et al. 2016). It was then further purified in a one-step chromatographic process, using a Q Sepharose Fast Flow® column (GE Healthcare Life Sciences). LsAA9A was prepared as described previously (Simmons et al. 2017). The LPMOs were Cu-loaded with sub-stoichiometric amounts of copper, to minimize the amount of free copper in the assay. Holo-TaAA9A and Holo-LsAA9A were prepared by incubating TaAA9A and LsAA9A overnight at 4 °C with 1:0.75 sub-stoichiometric CuSO4. The supplied TtAA9E was further purified and Cu-loaded as described previously (Singh et al. 2019).
Cellulose degradation assays
All hydrolysis experiments were carried out in 50 mM Citrate/Phosphate buffer (pH 6), in 96-well microtitre plates. Experiments to determine the degree of synergy were conducted at a fixed total enzyme concentration of 1 µM, while stepwise varying the relative amounts in pairs of enzymes, consisting of an LPMO and a cellulase. The activities of single enzymes were measured at the same enzyme concentrations as those used for the single components in the synergy assays. Two cellulose concentrations were used for each type of substrate: 2.5 g/L and 5 g/L Avicel, and 2.8 g/L and 5.6 g/L PASC. Substrate-loaded microtitre plates were equilibrated at 50 °C before the addition of prepared enzyme solutions and ascorbic acid to a final concentration of 1 mM. The plates were incubated at 50 °C for 1 h in an Eppendorf Thermomixer at 1100 rpm. The completed Avicel reaction mixture was then centrifuged (3 min, 3500 rpm) and the supernatant was collected. The reaction mixture from PASC hydrolysis was centrifuged through filter plates with 0.22 µm pore size (Millipore MultiScreenHTS, 3 min, 3500 rpm) and the supernatant was collected.
Enzymatic activity was assessed by quantifying soluble sugars with a reducing-end detection assay, employing para-hydroxybenzoic acid hydrazide (PAHBAH) (Lever 1973). Sixty µl of reaction supernatant was transferred to a 96-well PCR plate, and 85 µl of 15 mg/mL PAHBAH solution, prepared in 0.18 M potassium sodium tartrate buffer, was then added. The plate was incubated at 95 °C for 10 min in a thermal cycler (Eppendorf Mastercycler X50). One hundred µl of the mixture was then transferred to a 96-well microtitre plate and the absorbance was measured at 405 nm in a plate reader (Molecular Devices Spectramax i3). The amount of reducing ends was quantified using glucose standard curves (0–1 mM) present on each plate, after subtraction of the result obtained from a blank sample. Enzymatic activity is presented as glucose equivalents per second (µM/sec). Standard deviations for each sample were calculated from a minimum of three technical replicates.
Data analysis
The degree of synergy (DS) of the coupled enzyme mixtures was determined for each enzyme combination and concentration ratio, according to Eq. (1):
where \({\text{A}}_{\text{C}\text{e}\text{l}7+\text{L}\text{P}\text{M}\text{O}}\)is the measured activity of a two-component enzyme mixture of cellulase and LPMO, and \({(\text{A}}_{\text{C}\text{e}\text{l}7}+{\text{A}}_{\text{L}\text{P}\text{M}\text{O}})\) is the sum of the measured activities of the single components, at concentrations identical to those in the enzyme mixture.
Results and discussion
Elucidation of the underlying mechanisms of cellulase-LPMO synergy can facilitate the application of LPMOs in industrial settings. To this end, we investigated the ability of three LPMOs, with different modes of action: TaAA9A, LsAA9A and TtAA9E, to synergise with two of the main components in some commercial enzyme cocktails: non-reducing-end acting Cel6A and reducing-end acting Cel7A, from the fungus Trichoderma reesei. Synergy curves were obtained by systematically varying the molar ratio of pairs of enzymes (an LPMO and a cellulase), while keeping the total enzyme concentration constant. Such curves are particularly informative of the most favourable enzymatic ratios, under given conditions. Furthermore, the shapes of the synergistic activity curves can be instructive for the mode of synergy that is exhibited by the cellulase-LPMO mixtures.
The rate of catalysis was determined on amorphous cellulose (PASC) and microcrystalline cellulose (Avicel), which is about 50% crystalline. The synergy curves obtained from the enzymatic hydrolysis of Avicel and PASC are shown in Figs. 1 and 2, respectively. The activity of the enzymatic mixtures, containing both a cellulase and an LPMO, are plotted as a function of enzyme molar ratio (black symbols and lines), with the amount of cellulase increasing to the right, as specified on the abscissa. The activities of the single enzymes, at concentrations equal to the concentrations of the individual components in the mixtures, are also plotted (cellulase and LPMO shown in colour). The sum of the activities of the individual enzymes, i.e. the activity that would be observed if the two enzymes acted independently, is indicated by the dashed lines.
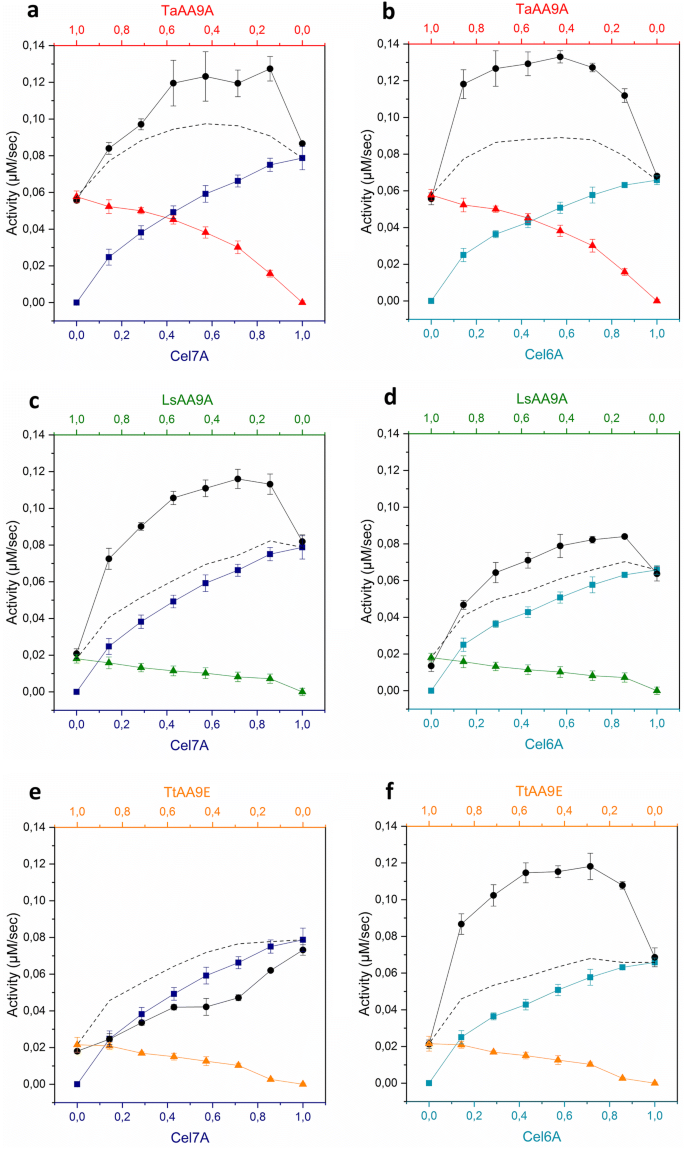
Synergy curves during degradation of Avicel (5 g/L) by mixtures of a cellulase and an LPMO. The activity of single enzymes are colour-coded – Right column: Cel7A (dark blue squares); Left column: Cel6A (cyan squares). a, b TaAA9A (red triangles); c, d LsAA9A (green triangles); e, f TtAA9E (orange triangles). Synergy curves of the enzyme mixtures at varied molar fractions (1 µM total enzyme) are shown in black circles. Dotted lines indicate the sum of the activities of the single enzymes. Experiments were performed at 50 °C for 1 h, at pH 6. Activity was assessed by a reducing-end assay. Error bars represent standard deviations obtained from at least three technical replicates
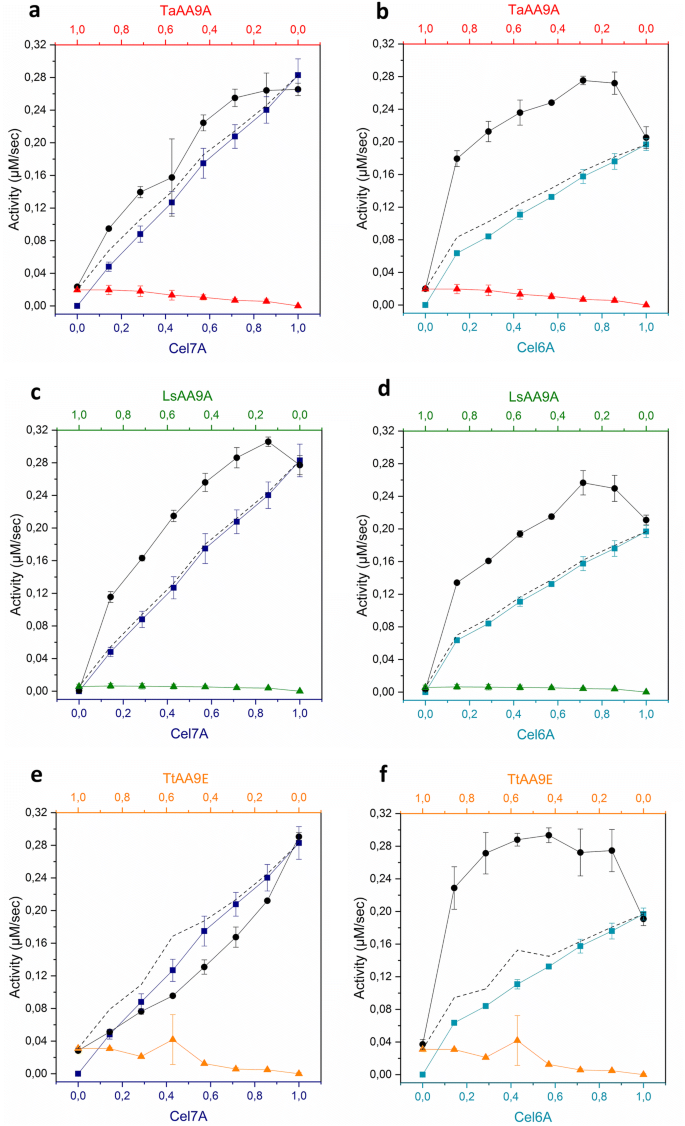
Synergy curves during degradation of PASC (5.6 g/L) by mixtures of a cellulase and an LPMO. The activity of single enzymes are colour-coded – Right column: Cel7A (dark blue squares); Left column: Cel6A (cyan squares). (a, b) TaAA9A (red triangles); (c, d) LsAA9A (green triangles); (e, f) TtAA9E (orange triangles). Synergy curves of the enzyme mixtures at varied molar fractions (1 µM total enzyme) are shown in black circles. Dotted lines indicate the sum of the activities of the single enzymes. Experiments were performed at 50 °C for 1 h, at pH 6. Activity was assessed by a reducing-end assay. Error bars represent standard deviations obtained from at least three technical replicates
Hydrolysis of cellulose by single enzymes
Cel6A and Cel7A exhibited significant differences in their ability to hydrolyse PASC and Avicel under the conditions tested, showing relatively low hydrolytic rates on Avicel (Fig. 1) and a higher preference for PASC (Fig. 2). This is in line with previous reports on the activities of the two enzymes (Zhang and Lynd 2004), and their more pronounced binding to amorphous cellulose (Christensen et al. 2018). Despite its higher affinity for the amorphous substrate, Cel6A is less efficient in finding attack sites on the cellulose surface compared to Cel7A (Christensen et al. 2018). In contrast, Cel7A is capable of binding productively to essentially all available binding sites.
The three LPMOs investigated, TaAA9A, LsAA9A and TtAA9E, were chosen for their differing substrate-specific activity and regioselectivity. The LPMOs exhibited very low or no activity on the amorphous substrate in the short timeframe of the synergy experiment (Fig. 2), with substrate conversion below 0.3% for the highest enzyme loadings (data not shown). However, TaAA9A exhibited a much higher catalytic rate on Avicel than the other two LPMOs (Fig. 1). This higher activity of TaAA9A on Avicel is consistent with earlier reports of the enzyme’s ability to act on this substrate (Quinlan et al. 2011). With longer incubation time (24 h), the differences between the regioselectivity of the LPMOs during PASC degradation can be observed and confirmed by high-performance anion-exchange chromatography (HPAEC) (Supplementary Fig. 1).
Cellulase-LPMO synergy
For 5 of the 6 enzyme mixtures, the observed synergistic activity on Avicel was higher than the sum of activities of the single enzymes. Both cellulases investigated were able to synergise with TaAA9A (Fig. 1a, b). However, reducing-end acting Cel7A synergised most efficiently with LsAA9A (Fig. 1c). The non-reducing-end acting cellulase, Cel6A, benefited most from the presence of the strictly C1-oxidizing LPMO, TtAA9E, as could be intuitively expected (Fig. 1f). In contrast, this LPMO appeared to have a negative effect on Cel7A (Fig. 1e). This is demonstrated by the lower activity of this enzyme mixture than the sum of the activities of the single components, as well as the Cel7A activity alone. This negative effect is discussed further below.
Cellulase-LPMO synergy on the amorphous substrate, PASC (Fig. 2), followed a similar trend to that observed during the degradation of the crystalline substrate, Avicel. Once again, the enzyme mixtures containing an LPMO and a cellulase showed higher activities than the sum of the activities of the single enzymes, with the exception of the Cel7A-TtAA9E mixture (Fig. 2e).
It has been shown that LPMO activity is important in increasing the conversion of cellulosic substrates by cellulases. Increased saccharification of pre-treated corn stover has been reported in a study, where the addition of TaAA9A to a cellulase mixture (or co-expression) allowed a 50% reduction in the enzyme load required to reach 90% cellulose hydrolysis (Harris et al. 2010). In another study on the synergistic effect of TaAA9A, Avicel conversion by Cel7A and Cel6A was increased by about 25% (from 18 to 25%) and 50% (from 5 to 8%), respectively, in the presence of the LPMO and gallate as reducing agent (Quinlan et al. 2011). This is in line with the more recent report of a 50% increase in the saccharification of bacterial microcrystalline cellulose by Trichoderma longibrachiatum Cel7A upon the addition of TrAA9A from Trichoderma reesei (Song et al. 2018). Therefore, the results presented here are qualitatively in accordance with past investigations. While these earlier works focused on prolonged reactions with high conversion, we used short contact time and the results may be interpreted as initial rates measurements (Kari et al. 2019). Hence, the synergy could be assessed with little interference from other factors, such as substrate modification, product inhibition and enzyme instability.
The cellulase-LPMO synergy observed in the present study can be illustrated well in terms of the degree of synergy (DS). The values of DS shown in Fig. 3 indicate that the mechanism of synergy between LPMOs and cellulases is both enzyme- and substrate-specific. The DS also depends strongly on the cellulase:LPMO ratio. The highly processive, reducing-end acting cellulase, Cel7A (Fig. 3, left), exhibited a similar DS with LsAA9A and TaAA9A on both substrates; a more pronounced concentration dependency being seen for LsAA9A. The highest synergy for Cel7A was observed with LsAA9A during PASC hydrolysis (DS of about 2 at a cellulase:LPMO ratio of 1.5:8.5 ). Similar values have been reported for Cel7A with the endo-acting enzyme Cel7B during the hydrolysis of Avicel (Olsen et al. 2017). The non-reducing-end cellulase, Cel6A, in combination with the three LPMOs investigated (Fig. 3, right), exhibited higher DS on PASC than on Avicel; the highest DS being seen at low cellulase:LPMO ratios. The highest synergy was observed during PASC hydrolysis by Cel6A and TtAA9E, reaching a DS of 2.5 at a cellulase:LPMO ratio of 3:7. As mentioned above, Cel6A adsorbs more strongly to amorphous cellulose than to crystalline cellulose, but is less efficient at finding suitable attack sites on the surface of the amorphous substrate (Christensen et al. 2018). This could explain why higher values of DS were observed at higher concentrations of LPMOs, the action of which may increase the density of productive binding sites available for Cel6A.
Degree of synergy between LPMOs and cellulases: (a, c) Cel7A, (b, d) Cel6A. DS is presented for Avicel (top) and PASC (bottom), at varied molar fractions, with LPMO components shown in colour: TaAA9A (red squares); LsAA9A (green circles); TtAA9E (orange triangles). DS was calculated using Eq. (1), from data presented in Figs. 1 and 2. Error bars represent propagated standard deviations from the synergy curves
The shape of the synergy curves could provide useful information on the type of synergy exhibited by the cellulase-LPMO mixtures. An example of this is the observation of an almost linear increase in activity with maximum at low LPMO concentrations, which is seen for combinations of the highly processive Cel7A with the mixed-oxidation LPMOs, TaAA9A and LsAA9A (Fig. 1a, c). Such curves can be indicative of pure endo-exo synergy (Boisset et al. 2001). According to the conventional understanding of endo-exo synergism, small amounts of endo-acting enzyme increase chain-end availability for the exo-acting enzyme. It has been proposed, however, that at low enzyme:substrate ratios, the synergy between endo- and exo-acting enzymes may stem from the ability of endolytic enzymes to alleviate the stalling of exo-cellulases (Jalak et al. 2012). Thus, endo-exo synergy stems from the different specificities of the enzymes to specific regions of the substrate.
Interestingly, the endo-exo shaped synergy curve was observed during hydrolysis of the amorphous substrate PASC by the mixture of Cel6A and TaAA9A (Fig. 2b), whereas this was not observed with the more crystalline Avicel (Fig. 1b). Cases where the highest substrate conversion is observed in the form of a plateau, could be explained by neither the LPMO nor the cellulase being limiting. Rather, the plateau-shaped curves might be rationalized by a synergistic action that relies on differences in the specificity for different conformations on the cellulose surface. This type of mechanism has been proposed previously for synergy between different endoglucanases (endo-endo synergy) (Klyosov 1990; Zhou and Ingram 2000) and different cellobiohydrolases (exo-exo synergy) (Badino et al. 2017).
LPMO regiospecificity affects cellulase synergy
As mentioned above, TtAA9E showed strong synergy with Cel6A on both cellulosic substrates (Fig. 3b, d). It is intuitive that Cel6A will benefit from the creation of new non-reducing chain ends by the action of the C1-oxidizing TtAA9E. In contrast, the presence or activity of this LPMO appeared to have a negative effect on the highly processive, reducing-end acting, Cel7A (Fig. 3a, c). This is evident from the reduced activity of the enzyme mixture, compared to the sum of single-component activities. This effect is seen as a DS of less than 1 on both substrates, which not only indicates lack of synergy between the enzymes, but a component of obstruction or inhibition during cellulose hydrolysis by the Cel7A-TtAA9E mixture.
The lack of synergy between Cel7A and other strictly C1-oxidizing LPMOs has been reported previously. In a study by Eibinger et al., treatment of a highly crystalline substrate with the LPMO NcAA9 from Neurospora crassa resulted in a subsequent reduction in Cel7A hydrolytic activity, suggesting an inhibitory effect (Eibinger et al. 2014). However, no inhibition was observed on Avicel. Significant inhibition of Cel7A has also been observed during the treatment of Avicel and PASC by MtLPMO9L from Myceliophthora thermophila (Zhou et al. 2019). These results, and the results of the present study, suggest that the inhibition of Cel7A by C1-regioselective LPMOs is substrate- and enzyme-specific.
The observed inhibition may stem from the affinity of Cel7A for C1-oxidized chain ends. Molecular dynamics simulations by Vermaas et al. have suggested that there is a potential for inhibition by C1-oxidized sugars in the product-binding site of Cel7A. Modelling the effects of oxidation on product affinity indicates that it is cellobionic acid, the most predominant C1-oxidation form in solution, for which Cel7A has the highest affinity in its product-binding site (Vermaas et al. 2015).
The negative effect of TtAA9E on Cel7A observed here is not substrate specific. The inhibition is observed for both Avicel and PASC. In contrast, a substrate-specific negative effect has been observed between a Cel6A and an endoglucanase Cel45A from Humicola insolens, during hydrolysis of Avicel (Andersen et al. 2008). Such substrate-specific inhibitory effect has been proposed to stem from enzyme competition for the same binding sites on the Avicel surface. However, studying the hydrolysis of oxidized cellulose by Cel7A and Cel6A has revealed the inability of Cel7A to perform binding and processive hydrolysis on a C1-oxidized chain end (Xu et al. 2009). Further investigations are, therefore, necessary in order to identify whether Cel7A-TtAA9E inhibition results from product inhibition by cellobionic acid, inability of Cel7A to thread C1-oxidized chain ends into its binding tunnel or cellulose surface obstruction by the LPMO. Current industrial cocktails rely on LPMOs for improved biomass degradation (Johansen 2016). However, the LPMO content of such cocktails is not known and there have been contrasting reports regarding the extent of C1/C4 oxidation present (Cannella et al. 2012; Müller et al. 2018). Cel7A makes up a significant portion of the enzymatic machinery in such cocktails. Therefore, the possibility of Cel7A inhibition warrants further investigation into the industrial implications and cocktail optimization.
Among the three LPMOs investigated, LsAA9A shows the highest DS when used in combination with Cel7A on PASC (Fig. 3c). It has previously been reported that the binding of LsAA9A to PASC is structurally similar to its binding to the soluble oligomer cellohexaose, as studied by electron paramagnetic resonance (EPR). Furthermore, it has been shown that LsAA9A performs oxidation strictly at the C4-position during oxidation of oligosaccharides (Frandsen et al. 2016). LsAA9A, therefore, exhibits regiospecificity defined by the substrate, which suggests that regiospecificity may be a less meaningful parameter for LPMO classification. The high DS of LsAA9A with Cel7A can be explained by the observation that during PASC hydrolysis, LsAA9A produces new chain ends via C4-oxidation, which are intuitively expected to be beneficial for the reducing-end acting cellulase, Cel7A (Supplementary Fig. 1).
Conclusions
In this study, we have investigated the synergy exhibited by binary combinations of three fungal LPMOs and two canonical cellulases, during cellulose saccharification at various molar ratios. The extent of the synergy exhibited by the cellulase-LPMO mixtures was found to be enzyme- and substrate-specific, while the inhibitory effect was not. The highest values of DS (2–2.5) were observed during saccharification of the amorphous PASC by Cel6A-LPMO mixtures. The highest degree of synergy during hydrolysis of the crystalline substrate Avicel was observed for mixtures of Cel7A and LsAA9A, and Cel6A and TtAA9E, while the highest activity was obtained for mixtures of TaAA9A with Cel6A and Cel7A. The contrasting results observed when using the reducing-end acting Cel7A and the C1-oxidizing TtAA9A suggest some degree of inhibition or competition. These findings warrant further investigation into the possible practical implications for industrial cellulase mixtures.
References
Andersen N, Johansen KS, Michelsen M et al (2008) Hydrolysis of cellulose using mono-component enzymes shows synergy during hydrolysis of phosphoric acid swollen cellulose (PASC), but competition on Avicel. Enzyme Microb Technol 42:362–370
Badino SF, Christensen SJ, Kari J et al (2017) Exo-exo synergy between Cel6A and Cel7A from Hypocrea jecorina: Role of carbohydrate binding module and the endo-lytic character of the enzymes. Biotechnol Bioeng 114:1639–1647
Boisset C, Pétrequin C, Chanzy H et al (2001) Optimized Mixtures of Recombinant Humicola insolens Cellulases for the Biodegradation of Crystalline Cellulose Claire. Biotechnol Bioeng 72:339–345
Cannella D, Hsieh CC, Felby C et al (2012) Production and effect of aldonic acids during enzymatic hydrolysis of lignocellulose at high dry matter content. Biotechnol Biofuels 5:1–10
Christensen SJ, Kari J, Badino SF et al (2018) Rate-limiting step and substrate accessibility of cellobiohydrolase Cel6A from Trichoderma reesei. FEBS J 285:4482–4493
Eibinger M, Ganner T, Bubner P et al (2014) Cellulose surface degradation by a lytic polysaccharide monooxygenase and its effect on cellulase hydrolytic efficiency. J Biol Chem 289:35929–35938
Frandsen KEH, Simmons TJ, Dupree P et al (2016) The molecular basis of polysaccharide cleavage by lytic polysaccharide monooxygenases. Nat Chem Biol 12:298–303
Harris PV, Welner D, McFarland KC et al (2010) Stimulation of lignocellulosic biomass hydrolysis by proteins of glycoside hydrolase family 61: Structure and function of a large, enigmatic family. Biochemistry 49:3305–3316
Hu J, Arantes V, Pribowo A et al (2014) Substrate factors that influence the synergistic interaction of AA9 and cellulases during the enzymatic hydrolysis of biomass. Energy Environ Sci 7:2308–2315
Jalak J, Kurašin M, Teugjas H, Väljamäe P (2012) Endo-exo Synergism in Cellulose Hydrolysis Revisited. J Biol Chem 287:28802–28815
Johansen KS (2016) Discovery and industrial applications of lytic polysaccharide mono-oxygenases. Biochem Soc Trans 44:143–149
Kari J, Christensen SJ, Andersen M et al (2019) A practical approach to steady-state kinetic analysis of cellulases acting on their natural insoluble substrate. Anal Biochem 586:113411
Klyosov AA (1990) Trends in Biochemistry and Enzymology of Cellulose Degradation. Biochemistry 29:10577–10585
Kracher D, Andlar M, Furtmüller PG, Ludwig R (2018) Active-site copper reduction promotes substrate binding of fungal lytic polysaccharide monooxygenase and reduces stability. J Biol Chem 293:1676–1687
Lever M (1973) Colorimetric and fluorometric carbohydrate determination with p-hydroxybenzoic acid hydrazide. Biochem Med 7:274–281
Mandels M, Reese ET (1965) Inhibition of cellulases. Annu Rev Phytopathol 3:85–102
Müller G, Várnai A, Johansen KS et al (2015) Harnessing the potential of LPMO-containing cellulase cocktails poses new demands on processing conditions. Biotechnol Biofuels 8:1–9
Müller G, Chylenski P, Bissaro B et al (2018) The impact of hydrogen peroxide supply on LPMO activity and overall saccharification efficiency of a commercial cellulase cocktail. Biotechnol Biofuels 11:1–17
Olsen JP, Borch K, Westh P (2017) Endo/exo-synergism of cellulases increases with substrate conversion. Biotechnol Bioeng 114:696–700
Quinlan RJ, Sweeney MD, Lo Leggio L et al (2011) Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc Natl Acad Sci 108:15079–15084
Scott BR, Zhi H, Jesper H et al (2016) Catalase improves saccharification of lignocellulose by reducing lytic polysaccharide monooxygenase-associated enzyme inactivation. Biotechnol Lett 38:425–434
Simmons TJ, Frandsen KEH, Ciano L et al (2017) Structural and electronic determinants of lytic polysaccharide monooxygenase reactivity on polysaccharide substrates. Nat Commun 8(1):1–12
Singh RK, Möllers KB, Russo DA et al (2019) Thermal Unfolding and Refolding of Lytic Polysaccharide Monooxygenase. R Soc Chem Adv 9(51):29734–29742
Song B, Li B, Wang X et al (2018) Real-time imaging reveals that lytic polysaccharide monooxygenase promotes cellulase activity by increasing cellulose accessibility. Biotechnol Biofuels 11:1–11
Väljamäe P, Sild V, Nutt A et al (1999) Acid hydrolysis of bacterial cellulose reveals different modes of synergistic action between cellobiohydrolase I and endoglucanase I. Eur J Biochem 266:327–334
Vermaas JV, Crowley MF, Beckham GT, Payne CM (2015) Effects of lytic polysaccharide monooxygenase oxidation on cellulose structure and binding of oxidized cellulose oligomers to cellulases. J Phys Chem B 119:6129–6143
Westh P, Kari J, Olsen J et al (2014) Cellobiohydrolase variants and polynucleotides encoding same. 27587
Xu F, Ding H, Tejirian A (2009) Detrimental effect of cellulose oxidation on cellulose hydrolysis by cellulase. Enzyme Microb Technol 45:203–209
Zhang YHP, Lynd LR (2004) Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol Bioeng 88:797–824
Zhou S, Ingram LO (2000) Synergistic hydrolysis of carboxymethyl cellulose and acid-swollen cellulose by two endoglucanases (CelZ and CelY) from Erwinia chrysanthemi. J Bacteriol 182:5676–5682
Zhou H, Li T, Yu Z et al (2019) A lytic polysaccharide monooxygenase from Myceliophthora thermophila and its synergism with cellobiohydrolases in cellulose hydrolysis. Int J Biol Macromol 139:570–576
Acknowledgements
This work was supported by the Novo Nordisk Foundation, Grant NO. NNF17SA0027704 to Katja Salomon Johansen.
Supporting information: Supplementary Fig. 1 – Comparison of cellulose degradation fingerprint of TaAA9A, LsAA9A and TtAA9E.
Supplementary material
Supplementary experiments were performed, in order to confirm the purity of the three LPMO enzyme preparations, as well as to compare directly their activity. Supplementary Fig. 1 shows a direct comparison of the activity of the three tested LPMOs – TaAA9A, LsAA9A and TtAA9E. PASC degradation experiments were carried out in 50 mM Citrate/Phosphate buffer (pH 6), with copper-loaded LPMO at a concentration of 4 µM, 0.4 g/L PASC and 4 mM ascorbic acid. Samples were incubated at 50 °C for 24 h in an Eppendorf Thermomixer at 1100 rpm. The reaction mixture was centrifuged through filter plates with 0.22 µm pore size (Millipore MultiScreenHTS, 3 min, 3500 rpm) and the supernatant was analysed by HPAEC-PAD.
TtAA9E’s strict C1-oxidation mechanism is confirmed by the lack of C4-oxidised oligosaccharides on the chromatogram (after 25 min). The proposed substrate-specific regioselectivity of LsAA9A can be observed by the presence of C4- and absence of C1-oxidised sugars. LsAA9A’s ability to cleave short-chain oligosaccharides is also evident by the presence of a larger glucose peak, compared to the other two LPMOs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. This article does not describe any studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tokin, R., Ipsen, J.Ø., Westh, P. et al. The synergy between LPMOs and cellulases in enzymatic saccharification of cellulose is both enzyme- and substrate-dependent. Biotechnol Lett 42, 1975–1984 (2020). https://doi.org/10.1007/s10529-020-02922-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-02922-0