Abstract
Background
Propofol, an extensively used intravenous anesthetic agents during cancer resection surgery, has been confirmed to execute anti-tumor effect on multiple cancers, including colorectal cancer (CRC). Although the role of propofol in CRC has been previously reported, its action mechanism remains poorly understood. This study further explored the biological function and underlying mechanism of propofol in CRC cells.
Methods
The cell proliferation, migration and invasion were assessed by methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay, wound healing assay and transwell assay, respectively. The expression levels microRNA-124-3p.1 (miR-124-3p.1) and AKT serine/threonine kinase 3 (AKT3) was analyzed by quantitative real-time polymerase chain reaction. Western blot assay was employed to measure the protein expression of MMP-9, Vimentin and Cyclin D1. The interaction between miR-124-3p.1 and AKT3 was predicted by TargetScan and confirmed by dual-luciferase reporter assay.
Results
Propofol inhibited CRC cell proliferation, migration and invasion. Knockdown of miR-124-3p.1 or AKT3 upregulation reversed the inhibitory effects of propofol on CRC cell proliferation and metastasis. Besides, AKT3 was a direct target of miR-124-3p.1 and its overexpression abated the anti-tumor effect of miR-124-3p.1 on CRC cell proliferation and metastasis.
Conclusion
Propofol inhibited CRC cell proliferation, migration and invasion by upregulating miR-124-3p.1 and downregulating AKT3, providing a new sight for propofol treatment of CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is one of the most frequent types of malignant cancer and with high morbidity and mortality, causing more than 600,000 deaths every year worldwide (Jemal et al. 2011; Siegel et al. 2014). Despite the decreasing incidence and mortality in several Western countries, CRC is still a main public health problem (Center et al. 2009). Many factors accelerate the occurrence of CRC, including smoking, excessive alcohol consumption, improperly dietary habits and lifestyle, overweight and obesity (Ferrari 2007). Although the treatment and early detection of CRC have been improved over the past few decades, the 5-year survival rate remains low (Siegel et al. 2013). CRC patients mainly die of metastasis, and most of them still have poor prognosis. Hence, it is urgent to search novel and more effective strategies for CRC treatment.
Propofol (2, 6-diisopropylphenol) is an intravenous anesthetic agent and widely used in various surgeries owing to its short effect, rapid recovery and little side effects (Chidambaran et al. 2015). Additionally, propofol also possesses anti-tumor effects on a large number of cancers, such as gastric cancer (Zhang et al. 2018), cervical cancer (Zhang 2015), lung cancer (Sun and Gao 2018), ovarian cancer (Huang et al. 2016), and breast cancer (Siddiqui 2005). In terms of CRC, Xu et al. indicated that propofol inhibited CRC cell proliferation (Xu et al. 2018). Considering the widespread clinical application of propofol, more explorations are needed to understand the biological functions and underlying mechanisms of propofol in the progression of CRC.
MicroRNAs (miRNAs), small (~ 22 nucleotides) non-coding RNA molecules, negatively regulate target genes expression through binding to the 3′ untranslated regions (3′UTR) of mRNA containing complementary sequences (Bartel 2009, 2004). Several miRNAs have been identified to be abnormally expressed in CRC, suggesting that these miRNAs may play critical roles in the occurrence and progression of CRC. For instance, miR-185 and miR-133b deregulation were closely linked to overall survival as well as metastasis in CRC (Akcakaya 2011). Moreover, miR-150 has been shown to be a biomarker related to prognosis and therapeutic outcome in CRC (Ma 2012). As for miR-124, it has been demonstrated to be aberrantly expressed in CRC (Sun et al. 2012; Zhang 2013a). Among these CRC-related miRNAs, miR-124 was confirmed to show the most significant difference between the CRC cell lines and normal epithelial colon cell line. However, there is no evidence to support whether propofol exerts anti-tumor effect on CRC via regulating miR-124-3p.1.
Identifying the target genes of miRNAs is so important to better understand their specific biological function (Yue et al. 2009). In the present study, TargetScan bioinformatics analysis (https://www.targetscan.org/vert_71/) showed that AKT3, a CRC-related gene, was one of the interesting target genes of miR-124-3p.1. The AKT kinase family consists of three members: AKT1, AKT2, and AKT3, which play crucial roles in multiple cellular transformation processes, including cell proliferation, apoptosis, and migration (Hers et al. 2011). AKT3 has been confirmed to be dysregulated in several cancers, including CRC (Stahl 2004; Grottke 2016; Fang et al. 2018). Nevertheless, the interaction between AKT3 and miR-124-3p.1 in CRC is still unclear.
In our research, RKO and HCT116 cells were treated with propofol to explore its biological functions in the progression of CRC. To elaborate the possible mechanism of anti-tumor effect of propofol in CRC cells, we measured the expression levels of miR-124-3p.1 and AKT3, and explored relationship among them in CRC cells. Our findings might offer a novel theoretical basis for further investigation of propofol in treatment CRC.
Materials and methods
Cell culture and transfection
The human CRC cell lines (RKO and HCT116) were bought from American Tissue Culture Collection (Manassas, VA, USA) and normal epithelial colon cell line (NCM460) was purchased from Incell Corporation (San Antonio, TX, USA). RKO and HCT116 cells were maintained in Dulbecco’s modified eagle medium (DMEM) and NCM460 cells were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA). The medium contained 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA), penicillin (100 U/mL) and streptomycin (100 mg/mL) (Gibco). Then all cells were cultivated at 37 °C under in a moist atmosphere with 5% CO2.
Genepharma Inc. (Shanghai, China) accomplished the synthesis of miR-124-3p.1 mimics (miR-124-3p.1), negative control (miR-NC), miR-124-3p.1 inhibitors (anti-miR-124-3p.1), negative control (anti-miR-NC), AKT3 overexpression vector (AKT3), and empty vector (vector). According to the recommendations, cell transfection was carried out using Lipofectamine 2000 (Invitrogen).
Cell proliferation assay
RKO and HCT116 cells were seeded in 96-well plates (5 × 103 cells/well). After treatment/transfection, methylthiazolyldiphenyl-tetrazolium bromide (MTT, 5 mg/mL, 20 μL, Beyotime Biotechnology, Shanghai, China) was added into the culture medium of each corresponding well and the cells were maintained in humidified incubator at 37 °C for 4 h. Subsequently, the mixed medium was gently removed and the corresponding wells were added with dimethyl sulfoxide solution (DMSO; 150 μL). After that, cell proliferation was evaluated by measuring the absorbance of each well under a Microplate Reader (Bio-Rad, Hercules, CA, USA) at 490 nm.
Transwell assay
Uncoated or Matrigel-coated transwell chamber containing 8 μm pores (Corning Incorporation, New York, NY, USA) was used for the migration or invasion assay, respectively. In Brief, after relevant treatment or/and transfection, RKO and HCT116 cells were suspended in serum-free medium (100 μL, DMEM) and placed in the upper chambers. The lower chamber was filled with 600 μL of DMEM medium containing 10% FBS. After incubation at 37 °C for 24 h, non-migrated and non-invaded cells from the upper chamber membranes were scraped off, and the migrated and invaded cells were fixed using 4% paraformaldehyde and stained using 0.1% crystal violet. The migrated and invaded cells were counted and photographed under a microscope (Olympus, Tokyo, Japan).
Wound healing assay
Migration ability was also determined using a wound-healing assay. Briefly, cells were seeded in 12-well plates and then treated with propofol or/and transfected with indicated vectors. Monolayers in the center of the wells were scraped a sterile pipette tips and washed with PBS. At 0 h and 24 h post-wounding, images were captured under a light microscope and the widths of each scratch wound were recorded.
Western blot assay
RIPA lysis buffer (Beyotime Biotechnology) containing protease inhibitors (Roche, Basel, Switzerland) was employed to extract the total protein from RKO and HCT116 cells after treatment/transfection. Total protein concentration was quantified using a bicinchoninic acid (BCA) Protein Assay Kit (Tanon, Shanghai, China). Total protein (40 µg) was separated using 8–10% SDS-PAGE and then transferred onto nitrocellulose membranes. After blocking, these membranes were probed using primary antibodies against MMP-9 (1:1000, ab73734, Abcam, Cambridge, MA, USA), Vimentin (1:1000, ab137321, Abcam), Cyclin D1(1:2000, ab226977, Abcam), AKT3 (1:2000, ab152157, Abcam), and GAPDH (1:5000, ab70699, Abcam) at 4 °C for 12–16 h. After washing, the membranes were probed with HRP-conjugated secondary antibody for 2 h. After that, imaging was performed using the Enhanced Chemiluminescence plus detection reagent (ECL; Tanon). Finally, the blots were imaged by VersaDoc™ MP 4000 (Bio-Rad) and were quantified by ImageJ software.
Quantitative real-time polymerase chain reaction (qRT-PCR)
TRIzol reagent (Invitrogen) was employed to isolate total RNAs from cells in accordance with the manufacturer’s protocols. Reverse transcription reaction was performed with TaqMan Reverse Transcription Kit or TaqMan microRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). To assess the miR-124-3p.1 and AKT3 expression, qPCR was performed with SYBR green detection kit (Toyobo, Tokyo, Japan) on CFX96™ Real-Time PCR Detection System (BioRad Laboratories, CA). miR-124-3p.1 (forward, 5′-ACACTCCAGCTGGGTAAGGCACGCGGTG-3′, and reverse, 5′-TGGTGTCGTGGAGTCG-3′); AKT3 (forward, 5′-TGAAGTGGCACACACTCTAACT-3′, and reverse, 5′- CCGCTCTCTCGACAAATGGA-3′); U6 (forward, 5′-CTCGCTTCGGCAGCACA-3′, and reverse, 5′-TGG TGT CGT GGA GTC G-3′); GAPDH (forward, 5′-GACTCATGACCACAGTCCATGC-3′, and reverse, 5′-AGAGGCAGGGATGATGTTCTG). The miR-124-3p.1 and AKT3 expression levels were evaluated using 2−ΔΔCt and normalized to U6 and GAPDH, respectively.
Dual-luciferase reporter assay
TargetScan was used for target prediction. The wild-type or mutant 3′-UTR of AKT3 containing the predicted miR-124-3p.1 binding sites was cloned and inserted into pGL3 plasmids (Promega, Madison, WI, USA) to generate WT-AKT3 or MUT-AKT3 reporter vector. RKO and HCT116 cells were co-transfected with WT-AKT3 or MUT-AKT3 and miR-124-3p.1 or miR-NC for 48 h. The Dual-Luciferase Reporter Assay System (Promega) was applied to assess luciferase activity. Renilla luciferase activity was used for normalization.
Statistical analysis
In this study, all experiments were repeated at least three times. Differences between two groups were assessed using a Student’s t-test. GraphPad version 6.0 software (GraphPad Software Inc., La Jolla, CA, USA) was used for statistical analyses. Statistically significant was indicated when P < 0.05.
Results
Propofol suppressed cell proliferation, migration and invasion in CRC cells
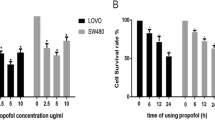
Firstly, the effects of propofol on CRC cell proliferation, migration and invasion were explored. Following treatment with different concentrations of propofol, the proliferation of RKO and HCT116 cells was measured by MTT assay. As presented in Fig. 1a, b, propofol suppressed the proliferation of RKO and HCT116 cells in dose- and time-dependent manners. Moreover, transwell assay indicated that propofol treatment repressed the migration and invasion of RKO and HCT116 cells in a dose-dependent manner (Fig. 1c, d). Wound healing assay further demonstrated that migratory areas of propofol were significantly smaller than those of control cells (Fig. 1e). Furthermore, the protein levels of MMP-9 and Vimentin (key factors in cancer cell migration and invasion) and Cyclin D1 (pro-proliferative protein) were measured by western blot. Result suggested that the protein levels of MMP-9, Vimentin and Cyclin D1 were markedly decreased in a dose-dependent way in propofol-treated RKO and HCT116 cells (Fig. 1f, g). These findings revealed that propofol could inhibit proliferation, migration and invasion of RKO and HCT116 cells.
Propofol inhibited CRC cell growth, migration and invasion. a, b MTT assay was performed to determine cell proliferation in RKO and HCT116 cells treated with different concentrations of propofol for 24 h, 48 h, or 72 h. c, d Transwell assay was used to detect cell migration and invasion in RKO and HCT116 cells exposed to various concentrations of propofol. e Cell mobility was investigated by wound healing assay in RKO and HCT116 cells exposed to various concentrations of propofol. f, g The protein levels of MMP-9, Vimentin and Cyclin D1 were measured in RKO and HCT116 cells treated with various concentrations of propofol by western blot assay. *P < 0.05
The expression of miR-124-3p.1 was upregulated in CRC cells treated with propofol
To explore the potential role of miR-124-3p.1 in CRC progression, the expression of miR-124-3p.1 was measured in CRC cells by qRT-PCR. Compared with NCM460 cells, RKO and HCT116 cells exhibited low expression of miR-124-3p.1 (Fig. 2a). Next, the effect of propofol on miR-124-3p.1 expression in RKO and HCT116 cells was evaluated by qRT-PCR. Results indicated that propofol treatment elevated the expression of miR-124-3p.1 in a dose-dependent way (Fig. 2b, c). These results proved that propofol enhanced the expression of miR-124-3p.1 in CRC cells.
Propofol upregulated the expression of miR-124-3p.1 in CRC cells. a The expression of miR-124-3p.1 was measured in cells (NCM460, RKO and HCT116) by qRT-PCR. b, c The abundance of miR-124-3p.1 was evaluated using qRT-PCR in RKO and HCT116 cells treated with various concentrations of propofol. *P < 0.05
Propofol suppressed CRC cell proliferation, migration and invasion by upregulating miR-124-3p.1
To confirm whether miR-124-3p.1 was required for propofol-mediated processes in CRC, RKO and HCT116 cells were transfected with anti-miR-124-3p.1 or anti-miR-NC and then treated with 8 μg/mL propofol. As shown in Fig. 3a and b, propofol treatment evidently enhanced the expression of miR-124-3p.1, while miR-124-3p.1 inhibitor transfection prominently decreased the expression of miR-124-3p.1 in RKO and HCT116 cells. MTT analysis displayed that cell proliferation was increased in propofol+anti-miR-124-3p.1 group at different time points compared to single propofol treatment group in RKO and HCT116 cells (Fig. 3c, d). Results showed that knockdown of miR-124-3p.1 attenuated the inhibitory effects of propofol on cell migration and invasion in RKO and HCT116 cells (Fig. 3e–j). Moreover, the protein levels of MMP-9, Vimentin and Cyclin D1 were apparently enhanced in propofol+anti-miR-124-3p.1 group relative to that in propofol+anti-miR-NC group (Fig. 3k, l). Taken together, these above results demonstrated that knockdown of miR-124-3p.1 could partly reverse the inhibitory effects of propofol on cell proliferation, migration and invasion in RKO and HCT116 cells.
Propofol suppressed CRC cell proliferation, migration and invasion by upregulating miR-124-3p.1. RKO and HCT116 cells were transfected with anti-miR-124-3p.1 or anti-miR-NC and then treated with 8 μg/mL propofol. a, b The expression of miR-124-3p.1 was assessed by qRT-PCR. c, d MTT assay was used to estimate cell proliferation. e–h The migration and invasion capacities of RKO and HCT116 cells were determined using transwell assay. i, g Cell migration was determined by wound healing assay. k, l The protein levels of MMP-9, Vimentin and Cyclin D1 were detected in RKO and HCT116 cells by western blot assay. *P < 0.05
AKT3 was a direct target of miR-124-3p.1 in CRC cells
To elucidate the underlying mechanism of miR-124-3p.1 in CRC cells, TargetScan bioinformatics analysis was employed to search for potential downstream targets of miR-124-3p.1. TargetScan showed that 3′-UTR of AKT3 contained the potential binding sites of miR-124-3p.1 (Fig. 4a). To verify that AKT3 was a direct target of miR-124-3p.1 and regulated by it, the AKT3 3′-UTR was amplified and inserted into a luciferase reporter vector. The dual-luciferase report assay indicated that overexpression of miR-124-3p.1 resulted in a great loss of luciferase activity in RKO and HCT116 cells transfected with AKT3-WT, while the luciferase activity was unaffected in AKT3-MUT group (Fig. 4b, c). Besides, the expression of miR-124-3p.1 was conspicuously increased in RKO and HCT116 cells transfected with miR-124-3p.1 mimics, suggesting that successful introduction of miR-124-3p.1 into RKO and HCT116 cells (Fig. 4d, e). Besides, accumulation of miR-124-3p.1 greatly downregulated the mRNA and protein expression of AKT3 in RKO and HCT116 cells, while miR-124-3p.1 knockdown showed an opposite effect (Fig. 4f–i). Collectively, these results suggested that AKT3 was a downstream target of miR-124-3p.1 in CRC cells.
AKT3 was a direct target of miR-124-3p.1. a The putative binding sites of miR-124-3p.1 within the 3′-UTR of WT-AKT3 (wild-type AKT3) are presented. b, c The luciferase activity was measured in RKO and HCT116 cells co-transfected with WT-AKT3 or MUT-AKT3 and miR-124-3p.1 mimics or miR-NC. d, e The expression of miR-124-3p.1 was determined using qRT-PCR in RKO and HCT116 cells transected with miR-124-3p.1 or miR-NC. f–i The mRNA and protein expression of AKT3 in RKO and HCT116 cells transfected with miR-NC, miR-124-3p.1, anti-miR-NC, or anti-miR-124-3p.1 were detected by qRT-PCR and western blot, respectively. *P < 0.05
Upregulation of AKT3 reversed the inhibitory effects of miR-124-3p.1 overexpression on CRC cell proliferation, migration and invasion
The qRT-PCR and western blot analysis were used to confirm the success of transduction. As displayed in Fig. 5a and b, the mRNA and protein expression of AKT3 were notably promoted in RKO and HCT116 cells transfected with AKT3 compared with those transfected with vector. To assess whether AKT3 could regulate the biological function of miR-124-3p.1 in CRC, RKO and HCT116 cells were transfected with miR-NC, miR-124-3p.1, miR-124-3p.1 + vector, or miR-124-3p.1+AKT3. MTT assay proved that addition of AKT3 alleviated the anti-proliferative effect of miR-124-3p.1 overexpression on proliferation of RKO and HCT116 cells (Fig. 5c, d). Moreover, high level of AKT3 reversed the suppressive effects of miR-124-3p.1 upregulation on migration and invasion of RKO and HCT116 cells (Fig. 5e–j). Besides, overexpression of AKT3 alleviated the miR-124-3p.1-mediated the inhibition of MMP-9, Vimentin and Cyclin D1 levels in RKO and HCT116 cells (Fig. 5k, l). In conclusion, these results indicated that upregulation of AKT3 could abate the inhibitory effects of miR-124-3p.1 overexpression on CRC cell proliferation, migration and invasion.
AKT3 reversed the inhibitory effects of miR-124-3p.1 on CRC cell growth, migration and invasion. a, b The mRNA and protein expression of AKT3 in RKO and HCT116 cells transfected with vector or AKLT3 were measured by qRT-PCR and western blot, respectively. c–j RKO and HCT116 cells were transfected with miR-NC, miR-124-3p.1, miR-124-3p.1 + vector, or miR-124-3p.1 + AKT3. c, d Cell proliferation of RKO and HCT116 cells was evaluated by MTT assay. e–h The migration and invasion abilities of RKO and HCT116 cells were determined using transwell assay. i, g Cell migration was assessed by wound healing assay. k, l Western blot assay was employed to measure the protein expression of MMP-9, Vimentin and Cyclin D1. *P < 0.05
Overexpression of AKT3 weakened the suppressive effects of propofol on cell proliferation, migration and invasion in CRC cells
To analyze the effects of AKT3 on propofol-induced CRC cell proliferation, migration and invasion, RKO and HCT116 cells were transfected with vector or AKT3 and subsequently exposed to propofol. The qRT-PCR and western blot analysis showed that mRNA and protein levels of AKT3 were obviously decreased in RKO and HCT116 cells treated with propofol, whereas the effects were abolished by addition of AKT3 (Fig. 6a, b). In addition, upregulation of AKT3 attenuated the inhibitory effects of propofol on cell proliferation, migration and invasion in RKO and HCT116 cells (Fig. 6c–j). Besides, AKT3 overexpression reversed the reductions of MMP-9, Vimentin and Cyclin D1 expression caused by propofol in RKO and HCT116 cells (Fig. 6k–l). Thus, our findings indicated that propofol suppressed CRC cell proliferation, migration and invasion by downregulating AKT3.
Propofol suppressed CRC cell proliferation, migration and invasion by downregulating AKT3. RKO and HCT116 cells were transfected with vector or AKT3 and subsequently exposed to propofol. a, b The mRNA and protein expression of AKT3 were determined by qRT-PCR and western blot, respectively. c, d Cell proliferation was evaluated using MTT assay. e–h Transwell assay was used to detect cell migration and invasion. i, j Wound healing assay was used to evaluate cell migration ability. k, l Western blot assay was conducted to assess the protein levels of MMP-9, Vimentin and Cyclin D1. *P < 0.05
Discussion
CRC is one of the most commonly diagnosed cancers in females and males (Torre et al. 2015). Propofol is frequently used for intravenous anesthetic and has anti-tumor effects on many cancers. In the present research, we presented that propofol could repress CRC cell proliferation, migration and invasion. In addition, we proved that propofol upregulated the abundance of miR-124-3p.1 and downregulated AKT3 expression. Moreover, we found that miR-124-3p.1 and AKT3 were involved in the anti-tumor function of propofol in CRC cell proliferation and metastasis.
Recent studies demonstrated that propofol not only possessed the advantages of various anesthetics, but also had a variety of non-anesthetic functions, such as antioxidant, anti-tumor, antiemetic, and neuroprotective effects (Vasileiou 2009; Jiang et al. 2018). In the present study, we showed that propofol obviously suppressed proliferation and metastasis of RKO and HCT116 cells. In addition, the pro-proliferative protein (Cyclin D1) and metastasis-related proteins (MMP-9 and Vimentin) were reduced by propofol treatment. Our results indicated that propofol had tumor suppressive effect in CRC, which was in line with the previous researches. For instance, Miao et al. indicated that propofol stimulation inhibited colon carcinoma cell invasion (Miao et al. 2010). Moreover, Ren et al. demonstrated that propofol treatment limited CRC cell proliferation (Ren and Zhang 2019).
Growing evidence proved that multiple miRNAs might be involved in the biological function of propofol in a variety of cancers, including miR-22 (Liu et al. 2016), miR-199a (Zhang et al. 2013b), miR-143 (Ye et al. 2014). MiR-124 has been confirmed to execute anti-tumor role in diverse types of cancers, such as gastric (Xia 2012), prostate cancer (Shi 2013), ovarian cancer (Zhang et al. 2013c). Additionally, Zhang et al. pointed out that miR-124 restrained CRC cell growth through downregulating STAT3 (Zhang 2013a). Nevertheless, whether miR-124-3p.1 was required for propofol-mediated processes in CRC has not yet been reported. Here, we observed that the miR-124-3p.1 level was reduced in CRC cells. Propofol stimulation enhanced the level of miR-124-3p.1. Moreover, miR-124-3p.1 downregulation abolished the inhibitory effects of propofol on CRC cell proliferation and metastasis. Our data revealed that propofol might exert anti-tumor effect on CRC cells via upregulating miR-124-3p.1.
To further explore the possible mechanism of anti-tumor function of propofol in CRC cells, the potential targets of miR-124-3p.1 were analyzed. TargetScan showed that 3′-UTR of AKT3 contained the potential binding sites of miR-124-3p.1. Subsequently, this prediction was validated through dual-luciferase reporter assay. AKT3, a tumor promoter, has been found to be associated with various cancer development and progression (Mo et al. 2016; Cristiano 2006). In recent, it was found that the abundance of AKT3 was increased in CRC cells and knockdown of AKT3 blocked CRC cell proliferation (Fang et al. 2018). In addition, Wang et al. revealed that upregulation of AKT3 accelerated CRC cell growth (Wang et al. 2018). In the current research, we proved that restoration of AKT3 could abate the suppressive effects of miR-124-3p.1 on CRC cell growth and metastasis. Furthermore, we showed that propofol treatment reduced the level of AKT3 in CRC cells, while the effect was abated by transduction of AKT3. Besides, upregulation of AKT3 weakened the suppressive effects of propofol on CRC cell proliferation and metastasis. Collectively, these results disclosed that propofol repressed CRC cell proliferation and metastasis by upregulating miR-124-3p.1 and downregulating AKT3.
Conclusion
Our study further validated the anti-tumor role of propofol in CRC cell proliferation and metastasis. Besides, we revealed that AKT3 was a downstream target of miR-124-3p.1. Furthermore, propofol exerted inhibitory effects on CRC cell proliferation and metastasis through modulating miR-124-3p.1/AKT3. This research might provide a novel theoretical basis for better study of propofol in the treatment of CRC.
References
Akcakaya P et al (2011) miR-185 and miR-133b deregulation is associated with overall survival and metastasis in colorectal cancer. Int J Oncol 39(3):11–318
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215
Center MM, Jemal A, Smith RA, Ward E (2009) Worldwide variations in colorectal cancer. CA Cancer J Clin 59:366–378
Chidambaran V, Costandi A, D’Mello A (2015) Propofol: a review of its role in pediatric anesthesia and sedation. CNS Drugs 29:543–563
Cristiano BE et al (2006) A specific role for AKT3 in the genesis of ovarian cancer through modulation of G2-M phase transition. Cancer Res 66:11718–11725
Fang Y, Liang X, Xu J, Cai X (2018) miR-424 targets AKT3 and PSAT1 and has a tumor-suppressive role in human colorectal cancer. Cancer Manage Res 10:6537
Ferrari P et al (2007) Lifetime and baseline alcohol intake and risk of colon and rectal cancers in the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer 121:2065–2072
Grottke A et al (2016) Downregulation of AKT3 increases migration and metastasis in triple negative breast cancer cells by upregulating S100A4. PLoS ONE 11:e0146370
Hers I, Vincent EE, Tavaré JM (2011) Akt signalling in health and disease. Cell Signal 23:1515–1527
Huang X, Teng Y, Yang H, Ma J (2016) Propofol inhibits invasion and growth of ovarian cancer cells via regulating miR-9/NF-κB signal. Braz J Med Biol Res. https://doi.org/10.1590/1414-431x20165717
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Jiang S, Liu Y, Huang L, Zhang F, Kang R (2018) Effects of propofol on cancer development and chemotherapy: potential mechanisms. Eur J Pharmacol 831:46–51
Liu Z, Zhang J, Hong G, Quan J, Zhang L, Yu M (2016) Propofol inhibits growth and invasion of pancreatic cancer cells through regulation of the miR-21/Slug signaling pathway. Am J Transl Res 8:4120
Ma Y et al (2012) miR-150 as a potential biomarker associated with prognosis and therapeutic outcome in colorectal cancer. Gut 61:1447–1453
Miao Y, Zhang Y, Wan H, Chen L, Wang F (2010) GABA-receptor agonist, propofol inhibits invasion of colon carcinoma cells. Biomed Pharmacother 64:583–588
Mo X, Cao Q, Liang H, Liu J, Li H, Liu F (2016) MicroRNA-610 suppresses the proliferation of human glioblastoma cells by repressing CCND2 and AKT3. Mol Med Rep 13:1961–1966
Ren YL, Zhang W (2019) Propofol promotes apoptosis of colorectal cancer cells via alleviating the suppression of lncRNA HOXA11-AS on miRNA let-7i. Biochem Cell Biol. https://doi.org/10.1139/bcb-2018-0235
Shi X-B et al (2013) Tumor suppressive miR-124 targets androgen receptor and inhibits proliferation of prostate cancer cells. Oncogene 32:4130
Siddiqui RA et al (2005) Anticancer properties of propofol-docosahexaenoate and propofol-eicosapentaenoate on breast cancer cells. Breast Cancer Res 7:R645
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30
Siegel R, DeSantis C, Jemal A (2014) Colorectal cancer statistics, 2014. CA Cancer J Clin 64:104–117
Stahl JM et al (2004) Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res 64:7002–7010
Sun H, Gao D (2018) Propofol suppresses growth, migration and invasion of A549 cells by down-regulation of miR-372. BMC Cancer 18:1252
Sun Y, Zhao X, Zhou Y, Hu Y (2012) miR-124, miR-137 and miR-340 regulate colorectal cancer growth via inhibition of the Warburg effect. Oncol Rep 28:1346–1352
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Vasileiou I et al (2009) Propofol: a review of its non-anaesthetic effects. Eur J Pharmacol 605:1–8
Wang YX, Zhu HF, Zhang ZY, Ren F, Hu YH (2018) MiR-384 inhibits the proliferation of colorectal cancer by targeting AKT3. Cancer Cell Int 18:124. https://doi.org/10.1186/s12935-018-0628-6
Xia J et al (2012) miR-124 inhibits cell proliferation in gastric cancer through down-regulation of SPHK1. J Pathol 227:470–480
Xu K, Tao W, Su Z (2018) Propofol prevents IL-13-induced epithelial–mesenchymal transition in human colorectal cancer cells. Cell Biol Int 42:985–993
Ye Z, Jingzhong L, Yangbo L, Lei C, Jiandong Y (2014) Propofol inhibits proliferation and invasion of osteosarcoma cells by regulation of microRNA-143 expression. Oncol Res Featur Preclin Clin Cancer Ther 21:201–207
Yue D, Liu H, Huang Y (2009) Survey of computational algorithms for microRNA target prediction. Curr Genomics 10:478–492
Zhang J et al (2013a) MiR-124 suppresses growth of human colorectal cancer by inhibiting STAT3. PLoS ONE 8:e70300
Zhang J, Zhang D, Wu G-Q, Feng Z-Y, Zhu S-M (2013b) Propofol inhibits the adhesion of hepatocellular carcinoma cells by upregulating microRNA-199a and downregulating MMP-9 expression. Hepatobiliary Pancreatic Dis Int 12:305–309
Zhang H, Wang Q, Zhao Q, Di W (2013c) MiR-124 inhibits the migration and invasion of ovarian cancer cells by targeting SphK1. J Ovarian Res 6:84
Zhang D et al (2015) Propofol promotes cell apoptosis via inhibiting HOTAIR mediated mTOR pathway in cervical cancer. Biochem Biophys Res Commun 468:561–567
Zhang W, Wang Y, Zhu Z, Zheng Y, Song B (2018) Propofol inhibits proliferation, migration and invasion of gastric cancer cells by up-regulating microRNA-195. Int J Biol Macromol 120:975–984
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Dong, W., Yang, H. et al. Propofol suppresses proliferation and metastasis of colorectal cancer cells by regulating miR-124-3p.1/AKT3. Biotechnol Lett 42, 493–504 (2020). https://doi.org/10.1007/s10529-019-02787-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02787-y