Abstract
Microcarriers are 100- to 300-micron support matrices that permit the growth of adherent cells in bioreactor systems. They have a larger surface area to volume ratio in comparison to single cell monolayers, enabling cost-effective cell production and expansion. Microcarriers are composed of a solid matrix that must be separated from expanded cells during downstream processing stages. The detachment method is chosen on the basis of several factors like cell type, microcarrier surface chemistry, cell confluency and degree of aggregation. The development of microcarriers with a range of physiochemical properties permit controlled cell and protein associations that hold utility for novel therapeutics. In this review, we provide an overview of the recent advances in microcarrier cell culture technology. We also discuss its significance as an ex vivo research tool and the therapeutic potential of newly designed microcarrier systems in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microcarriers are support matrices that enhance the growth of cells in bioreactor systems (Park et al. 2018; Scheffler et al. 2018). For cell expansion, cells are grown on the surface of small solid particles that have been suspended in growth medium to enable cell adherence (De Silva Thompson et al. 2019; YekrangSafakar et al. 2018). The discovery of microcarriers is not a recent event as their initial characterization dates back as far as 1967 in studies by van Wezel and colleagues (Van Wezel 1967). The recent advances in microcarriers relate to their range of designs and surface modifications that have extended their repertoire to a plethora of clinical and ex vivo applications. In their simplest form, microcarriers are 100- to 300-micron-beads that have sufficient density to maintain suspension during stirring. A number of microcarriers have been synthesized and commercially utilized, including glass, diethylaminoethyl (DEAE)-dextran, acrylamide, polystyrene, collagen, and alginate (O'Connor et al. 2017). Alginic acid, for example, is a widely used biomaterial for cell encapsulation and drug delivery. Cartilage cells cultured in an alginate carrier have the ability to synthesize an extracellular matrix (ECM) that mimics natural cartilage. These materials can be subject to further surface modifications to modulate their behaviours upon cell/protein attachment (Dias et al. 2017).
All classes of cultured animal cells, namely mammalian, avian, fish and insect cells, were successfully cultured on microcarriers (Kubis et al. 2016). Cells of broad histotypic origin, including primary cells, diploid cell strains, established or transformed cell lines, hybrid cell lines and tumor cells, can be cultured on microcarriers. Selection of the suitable microcarrier depends on the cell types and applications. In general, microcarriers reduce the time, expenditure, and complexity of the apparatus necessary for the routine propagation of anchorage-dependent cells (Tavassoli et al. 2018). This is exemplified by Synthemax® II microcarriers which create synthetic surfaces that promote stem cell adherence and expansion for long-term cultures (Pennington et al. 2015). Other commercially available microcarriers include alginate-based carriers (GEM, Global Cell Solutions), dextran-based carriers (Cytodex, GE Healthcare), collagen-based carriers (Cultispher, Percell), and polystyrene-based carriers (Plastic, SoloHill Engineering) (Tavassoli et al. 2018). These microcarriers differ in their porosity, density, dimensions and surface chemistry (summarized in Table 1). The range of applications of any given microcarrier is related to its chemical composition. With technological advances, the microcarrier field is rapidly expanding and exciting new applications continue to emerge (Fig. 1). These applications will be reviewed herein.
Microcarrier culture techniques and their advantages
Microcarriers can be made of synthetic or natural polymers. Most of the early microcarriers were synthetic polymer-based, including those based on poly(lactide-co-glycolide) (PLGA), polyhydroxyethylmethacrylate, acrylamide, polystyrene and polyurethane (Li et al. 2015a). Whilst microcarriers produced from synthetic polymers show high reproducibility, they tend to lack cell recognition sites, which limits their use in cell expansion applications (Yang et al. 2002). Recently, increasing focus has been placed on natural polymers and their derivatives, as they are easy to obtain and display biocompatibility (Wu et al. 2018). Examples of natural polymers used to fabricate microcarriers include Gelatin (GELIBEAD, Ventregel, and CultiSpher), collagen (Verax), cellulose (Cytopore), chitosan, alginic acid, etc. Examples of the utility of both classes of microcarriers are discussed in this review. On the basis of their physical properties, microcarriers are further subdivided into solid and liquid microcarriers. Among these, solid microcarriers possess the advantages of adherence and cell expansion (Table 1). In cell cultivation methods using liquid/liquid systems i.e., liquid microcarriers, cells adhere and grow at the interface between the culture media and hydrophobic liquids (Hanga et al. 2017). Perfluorocarbons are used as the hydrophobic liquids since they do not show adverse effects on cell growth and have high oxygen solubility (Pilarek et al. 2013). It has been demonstrated that the liquid/liquid systems are flexible (independent of vessel shape), fully scalable and do not require proteolytic enzymes, scaffolds or inserts typically employed for the 3D-culture of animal cells (Pilarek et al. 2013). In addition, the anchorage-dependent mammalian cell growth is not restricted by the confluence effect.
Microcarriers are usually cultured in stirred-tank bioreactors, but other support systems, such as rotating wall microgravity bioreactors, Wave bioreactors and fluidized-bed bioreactors, have also been employed (Shekaran et al. 2016). They possess the advantage of enhanced production capacity as the high surface area-to-volume ratios achieved lead to high cell yields (Alkhatib et al. 2017; Reibetanz et al. 2016). Microcarrier systems are flexible and surface areas can be adjusted to promote cell-growth. The homogeneity of the microcarrier systems permits the monitoring and control of parameters, such as pH, pO2, and nutrient and metabolite concentrations. Improved control of the system leads to more reproducible cell cultures, a key characteristic for commercial cell-based protocols (Jakob et al. 2016; Wang et al. 2015). Microcarriers also facilitate scalability through large-scale cultures using conventional technologies, including the stirred-tank bioreactors used for suspended mammalian cell cultures. They also allow scale-up via bead-to-bead cell transfer by adding fresh microcarriers, without the necessity for enzymatic dissociation. Compared to static culture, higher cell densities (number of cells per mL) in relatively low volumes are achieved due to their high surface-to-volume ratio. Hence, microcarrier technology minimizes laboratory and storage space as well as labor required for routine production, ultimately reducing costs (Jin et al. 2016; Kubis et al. 2016). The number of handling stages of cells (opening and closing of culture vessels) is also reduced, decreasing the risk of damage or media contamination. All these advantages highlight the application of microcarriers for a wide range of laboratory and commercial applications.
In recent years, recombinant therapeutic proteins have garnered intense research attention (O'Connor et al. 2017; Park et al. 2018; Scheffler et al. 2018). This has driven the design of microcarriers that maximize protein yields. Mammalian, microbial, and insect expression systems have been optimized for therapeutic proteins/vaccines (Gupta et al. 2019). High throughput devices, modern bioreactors and scale-out technology have enabled significant improvements in the final yields and product qualities obtained from mammalian-based culture systems since they allow consistent scalability and control of culture conditions. Computational tools and simulation models have also increased product yields. These advances are exemplified by improvements in vaccine production, evidenced by the large-scale (200 L scale) production of inactivated EV71 virus using a microcarrier-based suspension bioreactor system (Wu et al. 2015). The efficiency of this system is superior to the previously described roller-bottle vaccine production system, yielding approximately 2–3 times more EV71 vaccine within 70 days (Wu et al. 2015).
Microcarriers for disease treatment
Microcarriers continue to attract intense clinical interest due to their ability to repair tissue and bone defects (Kornmuller et al. 2017; Moradian et al. 2017; Saltz and Kandalam 2016; Wang et al. 2018b; Yan et al. 2018; Yu et al. 2017; Zhang et al. 2017, 2016; Zhou et al. 2019). In particular, injectable microcarriers have value as cell carriers to injured sites using only small incisions (Hoareau et al. 2018; Perteghella et al. 2017; Wang et al. 2018b; Yan et al. 2018; Yu et al. 2017; Zhang et al. 2017; Zhou et al. 2019). Due to its non-toxicity, ease of functionalization, biocompatibility and biodegradability, pullulan (PULL) is considered an ideal material for tissue engineering (Mocanu et al. 2014). Aydogdu and colleagues designed PULL microspheres to deliver cells for bone tissue engineering (Aydogdu et al. 2016). PULL was cross-linked using trisodium trimetaphosphate to enhance its stability. In addition, improved cell compatibility was achieved through the use of silk fibroin coating and surface biomimetic mineralization following treatment with simulated bodily fluids. Silk fibroin coating induced higher osteogenic property relative to uncoated and simulated body fluid-coated PULL microspheres. In vitro, the PULL microspheres displayed slow degradation rates and supported the formation of new bone tissue (Aydogdu et al. 2016).
In a novel approach, Kankala et al. fabricated poly(lactic-co-glycolic acid)-based highly open porous microspheres (HOPMs) using microfluidics to harbour proliferating skeletal myoblasts. Their feasibility was then evaluated for cell delivery in situ (Kankala et al. 2019). The HOPMs were 280–370 microns in size and had open pores of 10–80 microns that provided an environment favourable for cell arrangements into elongated morphologies with deposited ECM. This facilitated cell adhesion, proliferation, and myogenic differentiation in vitro. In vivo results in mice demonstrated enhanced vascularization and partial myoblast differentiation. Since the use of porogens and multistep processing may alter the polymer structure leading to cytocompatibility issues, the authors validated the biosafety of the HOPMs using cell cultures in vitro, hemocompatibility assays ex vivo and acute toxicity studies in vivo in nude mice.
Cultivation of mesenchymal stem cells (MSCs) on materials which induce osteogenic differentiation is a promising strategy in tissue engineering and regenerative medicine. In related studies, Goncharenko and co-workers investigated the proliferation and differentiation of MSCs cultured on fibroin microcarriers (Goncharenko et al. 2017). Effective cell proliferation was noted on the surface of the microcarriers in vitro, which enhanced mineralization (Goncharenko et al. 2017). A range of alginate microcarriers have also been reported as candidates to accommodate MSCs in 3D environments, particularly during craniofacial bone tissue engineering (Saltz and Kandalam 2016).
Microcarriers can deliver proteins, nucleic acids, and cells, and can promote both neuroprotection and neurorepair (Andre et al. 2016). Treatments for neurodegenerative disorders have been revolutionized by stem cells and neuronal progenitor transplants (Kandalam et al. 2017). For instance, Kandalam et al. integrated human marrow-isolated adult multilineage-inducible (MIAMI) stem cells and fibronectin-coated microcarriers into an injectable silanized-hydroxypropyl methylcellulose hydrogel (Kandalam et al. 2017). This delivery system displayed a prolonged release of brain-derived neurotrophic factor (BDNF) encapsulated in the microcarriers. In neurological disorders, the released BDNF and the augmented secretion of growth factors by the stem cells could have a synergistic effect on damaged neural tissues.
In a novel multidisciplinary approach, Moradian and colleagues used bone marrow-derived MSCs (BMSCs) that were engineered to overexpress neurotrophin-3, a known neuroprotective agent and potent stimulator of neuronal differentiation (Moradian et al. 2017). PLGA microcarriers were designed as injectable scaffolds and their surface was functionalized by collagen as a bioadhesive agent to enhance BMSC attachment. The overexpression of neurotrophin-3 using these microcarriers enhanced dopaminergic neuron differentiation and resulted in an elevated secretion of dopamine (Moradian et al. 2017). Thus, the combination of engineered BMSCs capable of growth factor overexpression, adhesion molecules and 3D structure in the same polymeric scaffold represents a potential therapeutic strategy for Parkinson’s disease.
Daviaud et al. reported the functional recovery of hemiparkinsonian rats following treatment with human MIAMI cells attached to neurotrophin-3-releasing pharmacologically-active PLGA microcarriers (Daviaud et al. 2015). The released neurotrophin-3 from the microcarriers markedly increased cell survival and neuronal differentiation, conferring neuroprotection in an ex vivo organotypic model of Parkinson’s disease. Furthermore, vascular endothelial growth factor A (VEGFA) was identified as the potential mediator of the neuroprotective effect of MIAMI cells.
Microcarriers with oxygen-delivering capacity are of interest to wound healing applications and molybdenum disulfide quantum dots have been integrated into responsive porous microcarriers to permit controllable oxygen-delivery to wounds (Liu et al. 2019). These specific gelatin methacryloyl porous microcarriers were produced from inverse opal microparticles decorated with hemoglobin. As the microcarriers were porous, they displayed good biocompatibility and oxygen delivery (Liu et al. 2019). An additional advantage was their photoresponsive oxygen release. These remarkable properties show clinical promise for future applications in wound healing and tissue engineering systems.
Microcarriers for cell expansion
In spite of potential cell-damaging effects by affecting critical cell membrane proteins (Baumann and Doyle 1979), enzymatic cell dissociation using proteases in combination with chelating agents is still the most common strategy due to optimum cell recovery and reliability for an extensive range of cells (Merten 2015). A number of advances for maximizing the efficiency of dissociation using recombinant proteolytic enzymes have been reported. With the aim of developing an animal-component-free enzymatic dissociation, Rourou et al. investigated TrypLE Select (recombinant trypsin) and Accutase as trypsin substitutes. Subsequently, they demonstrated that TrypLE Select and soybean trypsin inhibitor were efficient for in situ Vero cell detachment from Cytodex-1 microcarriers and enzyme deactivation in bioreactors, without any effect on cell growth and proliferation (Rourou et al. 2013). Furthermore, Caruso et al. employed TrypLE Express (recombinant rypsin) for the dissociation of MSCs from Cytodex-3 with high cell viability and no damaging effects on the cells (Caruso et al. 2014). Nienow et al. developed a scalable method based on a short period of intense agitation in the presence of trypsin–EDTA for harvesting human MSCs from microcarriers (Nienow et al. 2014). The overall harvesting efficiency was greater than 95% and the cells maintained all their typical attributes. This flexible protocol has the potential to be applied to various cell lines by adjusting the agitation rate and time as well as the enzyme and its concentration.
Allogeneic keratinocytes can be used as cell therapies for the treatment of burn injuries and epithelial barrier restoration (Levato et al. 2015). However, significant cell loss due to poor adhesion in the wound means more controllable transplantation methods are required. Adhesion can be enhanced using cell microcarriers embedded in a biodegradable scaffold. Martin and colleagues produced keratinocyte-laden microcarriers that retained their basal proliferative phenotype after rapid expansion (Martin et al. 2017). The cell-binding microcarriers were incorporated into collagen scaffolds prepared by plastic compression, which when cultured in vitro, enhanced cell proliferation and migration. Wound healing models showed that the cells retained their basal proliferative phenotype and improved epithelial barrier restoration.
Due to the importance of stem cell therapies, microcarriers that aid stem cell expansion and differentiation have been extensively developed. Microcarriers in bioreactor culture systems are one of the most promising advancements and several commercially available systems are now in active use. Stimulus-responsive microcarriers that can be controlled by field, temperature, and pH changes are also under development (Shi et al. 2017; Tavassoli et al. 2018; Wang et al. 2018a). The features of the microcarriers include morphology, geometry, size, composition, nanotopography, surface coating, surface functionalization and charge. As an example, to replace lost retinal cells for the treatment of blindness, Baakdhah and coworkers used microcarriers in hypoxic stirring bioreactors to achieve cell numbers suitable for differentiation and transplantation (Baakdhah and van der Kooy 2019). The system used a combination of FACTIII microcarriers in a hypoxic (5% O2) suspension stirring bioreactor. Moreover, up to ten-times greater tertiary retinal stem cell yields were noted compared to normoxic static T-flask cultures (p = 0.02). The authors hypothesized that hypoxia activated Notch signaling and its downstream Hes cascade through stabilization of HIF1-α.
Classical adherent cell expansion methods using 2D monolayer flasks are limited by their lack of scalability, labor-intensive handling and enzymatic cell harvesting. To address these drawbacks, Lee et al. designed a series of alginate/PEG network microcarriers containing disulfide bonds which support the attachment and proliferation of human umbilical cord blood MSCs (Li et al. 2016). Following expansion, the cells could be detached from the microcarriers after the addition of l-cysteine as a reducing agent to cleave the disulfide crosslinkage, decreasing cell damage in response to proteolytic enzyme treatment. In similar studies, Shekaran and co-workers cultured human early MSCs (heMSCs) on biodegradable polycaprolactone microcarriers that were coated with ECM (a combination of fibronectin and poly-l-lysine) to overcome the aforementioned challenges (Shekaran et al. 2016). Following in vitro osteogenic induction, the heMSCs displayed ~ 68% higher levels of Ca2+ deposition compared to monolayer culture. In mouse ectopic mineralization models, similar improvements in bone mass were observed for monolayer-expanded and microcarrier-bound heMSC implants at 16 weeks post-implantation. The polycaprolactone microcarriers were not degraded at 16 weeks post-implantation since polycaprolactone degrades over 3–4 years. Moreover, the pores formed between the spherical microcarriers supported neovascularization in vivo, which indicated that they function similar to porous scaffolds. Therefore, these biodegradable microcarrier cultures could be employed for the large-scale expansion of heMSCs and can be delivered in vivo, eliminating the need for damaging cell harvesting procedures and for scaffolds for cell delivery (Shekaran et al. 2016).
It should be noted that the use of microcarriers to expand cells has not always been successful. The epithelium of the stomach encompasses multipotent SCs which differentiate into multiple cell lineages that produce hormones and peptides required for gastric function. The 3D culture of SCs enhances their differentiation. Alkhatib and colleagues designed disk-like immobile HD silicone-rubber microcarriers to minimize toxicity and enhance mouse gastric stem (mGS) cell expansion (Alkhatib et al. 2017). Several parameters that influence initial cell attachment were identified, including the cell inoculum, serum concentrations, and the speed of agitation. While the microcarrier permitted the attachment and growth of mGSs, they did not support the functional differentiation of these cells; whether this phenomenon is specific to this cell type warrants further investigation in a range of related cell models.
Cell expansion using microcarriers in suspension stirring bioreactors can also be performed through bead-to-bead cell transfer, which allows cells to migrate from confluent microcarriers onto fresh microcarriers without the need for enzymes and degradable carriers. This process involves either adding fresh microcarriers to already confluent microcarriers or transferring confluent microcarriers to a new bioreactor containing fresh microcarriers (Merten 2015). In high-cell density cultures, bead-to-bead transfer aids the augmentation of the microcarrier concentration and hence available culture surfaces for surface-adherent cells. Direct bead-to-bead transfer was demonstrated for several continuous cell lines, such as BHK-21, CHO, hMSCs, Vero and MDCK cells (Baakdhah and van der Kooy 2019; Rafiq et al. 2018). However, this method is limited by the mobility of cells and can lead to microcarrier aggregation. For example, human fibroblasts and hepatocytes are usually unable to undergo bead-to-bead transfer. The propensity of cell transfer between microcarriers can be increased by allowing the culture to remain static for prolonged periods, with occasional stirring to eliminate microcarrier aggregation (Merten 2015).
Conclusions and future perspectives
Microcarrier technology offers the advantages of culturing greater numbers of functional cells and maintaining their specific phenotypes, as well as being a source of cell therapy and tissue engineering. Engineered microcarrier culture systems that promote adherent cell growth represent a new paradigm in cell culture and tissue repair. The large surface area of the microcarriers enables the enhanced expansion of anchorage-dependent cells and proteins, with a range of therapeutic benefits. However, despite clear progress, several challenges remain. Due to the higher cell density, cell enumeration and harvesting in microcarrier cultures are difficult. Cells attached to the microcarriers often remain exposed to harmful adjoining surfaces, and cells growing across rounded surfaces show altered morphologies over time. Several advancements have been made to overcome these issues and expand the microcarrier repertoire, including physical immobilization and chemical modifications. Physical immobilization uses coating materials without altering the surface structure of the microcarrier. This endows microcarriers with the ability to simultaneously up-regulate cell functions and support cell proliferation. Surface functionalization is an effective strategy to overcome microcarriers’ inability to specifically interact with cells like natural ECM does. Recently, technologies to engineer the shape, nanotopography and functionality of microcarriers through surface modifications have expanded this field. Smart external stimuli-responsive materials have shown promise in cell cultures and could be exploited for the design of novel microcarriers. Furthermore, the development of cost-effective biodegradable microcarriers is essential for future applications.
References
Alkhatib R, Hilal-Alnaqbi A, Naciri M, Al-Majmaie R, Saseedharan P, Karam SM, Al-Rubeai M (2017) 3D culture of mouse gastric stem cells using porous microcarriers. Front Biosci (Schol Ed) 9:172–179
Andre EM, Passirani C, Seijo B, Sanchez A, Montero-Menei CN (2016) Nano and microcarriers to improve stem cell behaviour for neuroregenerative medicine strategies: application to Huntington's disease. Biomaterials 83:347–362. https://doi.org/10.1016/j.biomaterials.2015.12.008
Aydogdu H, Keskin D, Baran ET, Tezcaner A (2016) Pullulan microcarriers for bone tissue regeneration. Mater Sci Eng C Mater Biol Appl 63:439–449. https://doi.org/10.1016/j.msec.2016.03.002
Baakdhah T, van der Kooy D (2019) Expansion of retinal stem cells and their progeny using cell microcarriers in a bioreactor. Biotechnol Prog. https://doi.org/10.1002/btpr.2800
Baumann H, Doyle D (1979) Effect of trypsin on the cell surface proteins of hepatoma tissue culture cells. Characterization of a carbohydrate-rich glycopeptide released from a calcium binding membrane glycoprotein. J Biol Chem 254:3935–3946
Caruso SR, Orellana MD, Mizukami A et al (2014) Growth and functional harvesting of human mesenchymal stromal cells cultured on a microcarrier-based system. Biotechnol Prog 30:889–895. https://doi.org/10.1002/btpr.1886
Daviaud N, Garbayo E, Sindji L, Martinez-Serrano A, Schiller PC, Montero-Menei CN (2015) Survival, differentiation, and neuroprotective mechanisms of human stem cells complexed with neurotrophin-3-releasing pharmacologically active microcarriers in an ex vivo model of Parkinson's disease. Stem Cells Transl Med 4:670–684. https://doi.org/10.5966/sctm.2014-0139
De Silva Thompson D, Peticone C, Burova I et al (2019) Assessing behaviour of osteoblastic cells in dynamic culture conditions using titanium-doped phosphate glass microcarriers. J Tissue Eng 10:2041731419825772. https://doi.org/10.1177/2041731419825772
Derakhti S, Safiabadi-Tali SH, Amoabediny G, Sheikhpour M (2019) Attachment and detachment strategies in microcarrier-based cell culture technology: A comprehensive review. Mater Sci Eng C Mater Biol Appl 103:109782. https://doi.org/10.1016/j.msec.2019.109782
Dias AD, Elicson JM, Murphy WL (2017) Microcarriers with synthetic hydrogel surfaces for stem cell expansion. Adv Healthcare Mater. https://doi.org/10.1002/adhm.201700072
Goncharenko AV, Kotlyarova MS, Moisenovich AM et al (2017) Osteogenic differentiation of mouse bone marrow stromal cells on fibroin microcarriers. Dokl Biochem Biophys 477:345–348. https://doi.org/10.1134/S1607672917060011
Gupta SK, Dangi AK, Smita M, Dwivedi S, Shukla P (2019) Chapter 11—effectual bioprocess development for protein production. In: Shukla P (ed) Applied microbiology and bioengineering. Academic Press, New York, pp 203–227. https://doi.org/10.1016/B978-0-12-815407-6.00011-3
Hanga MP, Murasiewicz H, Pacek AW, Nienow AW, Coopman K, Hewitt CJ (2017) Expansion of bone marrow-derived human mesenchymal stem/stromal cells (hMSCs) using a two-phase liquid/liquid system. J Chem Technol Biotechnol 92:1577–1589. https://doi.org/10.1002/jctb.5279
Hoareau L, Fouchet F, Planesse C et al (2018) Combined therapy for critical limb ischaemia: biomimetic PLGA microcarriers potentiates the pro-angiogenic effect of adipose tissue stromal vascular fraction cells. J Tissue Eng Regen Med 12:1363–1373. https://doi.org/10.1002/term.2667
Jakob PH, Kehrer J, Flood P et al (2016) A 3-D cell culture system to study epithelia functions using microcarriers. Cytotechnology 68:1813–1825. https://doi.org/10.1007/s10616-015-9935-0
Jin GZ, Park JH, Wall I, Kim HW (2016) Isolation and culture of primary rat adipose derived stem cells using porous biopolymer microcarriers. Tissue Eng Regen Med 13:242–250. https://doi.org/10.1007/s13770-016-0040-z
Kandalam S, Sindji L, Delcroix GJ et al (2017) Pharmacologically active microcarriers delivering BDNF within a hydrogel: novel strategy for human bone marrow-derived stem cells neural/neuronal differentiation guidance and therapeutic secretome enhancement. Acta Biomater 49:167–180. https://doi.org/10.1016/j.actbio.2016.11.030
Kankala RK, Zhao J, Liu CG et al (2019) Highly porous microcarriers for minimally invasive in situ skeletal muscle cell delivery. Small. https://doi.org/10.1002/smll.201901397
Kornmuller A, Brown CFC, Yu C, Flynn LE (2017) Fabrication of extracellular matrix-derived foams and microcarriers as tissue-specific cell culture and delivery platforms. J Vis Exp. https://doi.org/10.3791/55436
Kubis HP, Scheibe RJ, Decker B, Hufendiek K, Hanke N, Gros G, Meissner JD (2016) Primary skeletal muscle cells cultured on gelatin bead microcarriers develop structural and biochemical features characteristic of adult skeletal muscle. Cell Biol Int 40:364–374. https://doi.org/10.1002/cbin.10565
Levato R, Planell JA, Mateos-Timoneda MA, Engel E (2015) Role of ECM/peptide coatings on SDF-1alpha triggered mesenchymal stromal cell migration from microcarriers for cell therapy. Acta Biomater 18:59–67. https://doi.org/10.1016/j.actbio.2015.02.008
Li B, Wang X, Wang Y et al (2015a) Past, present, and future of microcarrier-based tissue engineering. J Orthop Translat 3:51–57. https://doi.org/10.1016/j.jot.2015.02.003
Li P, Liu F, Wu C et al (2015b) Feasibility of human hair follicle-derived mesenchymal stem cells/CultiSpher((R))-G constructs in regenerative medicine. Cell Tissue Res 362:69–86. https://doi.org/10.1007/s00441-015-2182-z
Li C, Qian Y, Zhao S, Yin Y, Li J (2016) Alginate/PEG based microcarriers with cleavable crosslinkage for expansion and non-invasive harvest of human umbilical cord blood mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl 64:43–53. https://doi.org/10.1016/j.msec.2016.03.089
Liu Y, Zhao X, Zhao C, Zhang H, Zhao Y (2019) Responsive porous microcarriers with controllable oxygen delivery for wound healing. Small 15:e1901254. https://doi.org/10.1002/smll.201901254
Martin YH, Jubin K, Smalley S, Wong JPF, Brown RA, Metcalfe AD (2017) A novel system for expansion and delivery of human keratinocytes for the treatment of severe cutaneous injuries using microcarriers and compressed collagen. J Tissue Eng Regen Med 11:3124–3133. https://doi.org/10.1002/term.2220
Merten O-W (2015) Advances in cell culture: anchorage dependence. Philos Trans R Soc Lond B Biol Sci 370:20140040–20140040. https://doi.org/10.1098/rstb.2014.0040
Mocanu G, Nichifor M, Picton L, About-Jaudet E, Le Cerf D (2014) Preparation and characterization of anionic pullulan thermoassociative nanoparticles for drug delivery. Carbohyd Polym 111:892–900. https://doi.org/10.1016/j.carbpol.2014.05.037
Moradian H, Keshvari H, Fasehee H, Dinarvand R, Faghihi S (2017) Combining NT3-overexpressing MSCs and PLGA microcarriers for brain tissue engineering: a potential tool for treatment of Parkinson's disease. Mater Sci Eng C Mater Biol Appl 76:934–943. https://doi.org/10.1016/j.msec.2017.02.178
Nienow AW, Rafiq QA, Coopman K, Hewitt CJ (2014) A potentially scalable method for the harvesting of hMSCs from microcarriers. Biochem Eng J 85:79–88. https://doi.org/10.1016/j.bej.2014.02.005
O'Connor C, Steichen S, Peppas NA (2017) Student award for outstanding research winner in the undergraduate category for the 2017 society for biomaterials annual meeting and exposition, April 5–8, 2017, minneapolis, minnesota: development and characterization of stimuli-responsive hydrogel microcarriers for oral protein delivery. J Biomed Mater Res A 105:1243–1251. https://doi.org/10.1002/jbm.a.36030
Park J, Kwon S, Hwang NS, Kang BJ (2018) Clinical application of bone morphogenetic protein-2 microcarriers fabricated by the cryopolymerization of gelatin methacrylate for the treatment of radial fracture in two dogs. In Vivo 32:575–581. https://doi.org/10.21873/invivo.11278
Pennington BO, Clegg DO, Melkoumian ZK, Hikita ST (2015) Defined culture of human embryonic stem cells and xeno-free derivation of retinal pigmented epithelial cells on a novel, synthetic substrate. Stem Cells Transl Med 4:165–177. https://doi.org/10.5966/sctm.2014-0179
Perteghella S, Martella E, de Girolamo L et al (2017) Fabrication of innovative silk/alginate microcarriers for mesenchymal stem cell delivery and tissue regeneration. Int J Mol Sci. https://doi.org/10.3390/ijms18091829
Pilarek M, Grabowska I, Ciemerych MA, Dąbkowska K, Szewczyk KW (2013) Morphology and growth of mammalian cells in a liquid/liquid culture system supported with oxygenated perfluorodecalin. Biotech Lett 35:1387–1394. https://doi.org/10.1007/s10529-013-1218-2
Rafiq QA, Ruck S, Hanga MP et al (2018) Qualitative and quantitative demonstration of bead-to-bead transfer with bone marrow-derived human mesenchymal stem cells on microcarriers: utilising the phenomenon to improve culture performance. Biochem Eng J 135:11–21. https://doi.org/10.1016/j.bej.2017.11.005
Reibetanz U, Hubner D, Jung M, Liebert UG, Claus C (2016) Influence of growth characteristics of induced pluripotent stem cells on their uptake efficiency for layer-by-layer microcarriers. ACS Nano 10:6563–6573. https://doi.org/10.1021/acsnano.6b00999
Rourou S, Riahi N, Majoul S, Trabelsi K, Kallel H (2013) Development of an in situ detachment protocol of Vero cells grown on Cytodex1 microcarriers under animal component-free conditions in stirred bioreactor. Appl Biochem Biotechnol 170:1724–1737. https://doi.org/10.1007/s12010-013-0307-y
Saltz A, Kandalam U (2016) Mesenchymal stem cells and alginate microcarriers for craniofacial bone tissue engineering: a review. J Biomed Mater Res A 104:1276–1284. https://doi.org/10.1002/jbm.a.35647
Sart S, Agathos SN, Li Y (2013) Engineering stem cell fate with biochemical and biomechanical properties of microcarriers. Biotechnol Prog 29:1354–1366. https://doi.org/10.1002/btpr.1825
Scheffler K, Claus C, Stanifer ML, Boulant S, Reibetanz U (2018) Reversible fusion proteins as a tool to enhance uptake of virus-functionalized LbL microcarriers. Biomacromol 19:3212–3223. https://doi.org/10.1021/acs.biomac.8b00360
Shekaran A, Lam A, Sim E et al (2016) Biodegradable ECM-coated PCL microcarriers support scalable human early MSC expansion and in vivo bone formation. Cytotherapy 18:1332–1344. https://doi.org/10.1016/j.jcyt.2016.06.016
Shi Y, Ma C, Du Y, Yu G (2017) Microwave-responsive polymeric core–shell microcarriers for high-efficiency controlled drug release. J Mater Chem B 5:3541–3549. https://doi.org/10.1039/C7TB00235A
Tavassoli H, Alhosseini SN, Tay A, Chan PPY, Weng Oh SK, Warkiani ME (2018) Large-scale production of stem cells utilizing microcarriers: a biomaterials engineering perspective from academic research to commercialized products. Biomaterials 181:333–346. https://doi.org/10.1016/j.biomaterials.2018.07.016
Van Wezel AL (1967) Growth of cell-strains and primary cells on micro-carriers in homogeneous culture. Nature 216:64–65. https://doi.org/10.1038/216064a0
Wang J, Cheng Y, Yu Y, Fu F, Chen Z, Zhao Y, Gu Z (2015) Microfluidic generation of porous microcarriers for three-dimensional cell culture. ACS Appl Mater Interfaces 7:27035–27039. https://doi.org/10.1021/acsami.5b10442
Wang J, Chen G, Zhao Z, Sun L, Zou M, Ja Ren, Zhao Y (2018a) Responsive graphene oxide hydrogel microcarriers for controllable cell capture and release. Sci China Mater 61:1314–1324. https://doi.org/10.1007/s40843-018-9251-9
Wang Y, Yuan X, Yu K et al (2018b) Fabrication of nanofibrous microcarriers mimicking extracellular matrix for functional microtissue formation and cartilage regeneration. Biomaterials 171:118–132. https://doi.org/10.1016/j.biomaterials.2018.04.033
Wu CY, Lin YW, Kuo CH et al (2015) inactivated enterovirus 71 vaccine produced by 200-L scale serum-free microcarrier bioreactor system provides cross-protective efficacy in human SCARB2 transgenic mouse. PLoS ONE 10:e0136420. https://doi.org/10.1371/journal.pone.0136420
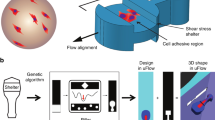
Wu CY, Stoecklein D, Kommajosula A, Lin J, Owsley K, Ganapathysubramanian B, Di Carlo D (2018) Shaped 3D microcarriers for adherent cell culture and analysis. Microsyst Nanoeng 4:21. https://doi.org/10.1038/s41378-018-0020-7
Yan S, Xia P, Xu S, Zhang K, Li G, Cui L, Yin J (2018) Nanocomposite porous microcarriers based on strontium-substituted HA-g-poly(gamma-benzyl-l-glutamate) for bone tissue engineering. ACS Appl Mater Interfaces 10:16270–16281. https://doi.org/10.1021/acsami.8b02448
Yang J, Shi G, Bei J et al (2002) Fabrication and surface modification of macroporous poly(L-lactic acid) and poly(L-lactic-co-glycolic acid) (70/30) cell scaffolds for human skin fibroblast cell culture. J Biomed Mater Res 62:438–446. https://doi.org/10.1002/jbm.10318
YekrangSafakar A, Acun A, Choi JW, Song E, Zorlutuna P, Park K (2018) Hollow microcarriers for large-scale expansion of anchorage-dependent cells in a stirred bioreactor. Biotechnol Bioeng 115:1717–1728. https://doi.org/10.1002/bit.26601
Yu C, Kornmuller A, Brown C, Hoare T, Flynn LE (2017) Decellularized adipose tissue microcarriers as a dynamic culture platform for human adipose-derived stem/stromal cell expansion. Biomaterials 120:66–80. https://doi.org/10.1016/j.biomaterials.2016.12.017
Zhang Z, Eyster TW, Ma PX (2016) Nanostructured injectable cell microcarriers for tissue regeneration. Nanomedicine (Lond) 11:1611–1628. https://doi.org/10.2217/nnm-2016-0083
Zhang S, Zhou M, Ye Z, Zhou Y, Tan WS (2017) Fabrication of viable and functional pre-vascularized modular bone tissues by coculturing MSCs and HUVECs on microcarriers in spinner flasks. Biotechnol J. https://doi.org/10.1002/biot.201700008
Zhou A, Ye Z, Zhou Y, Tan WS (2019) Bioactive poly(epsilon-caprolactone) microspheres with tunable open pores as microcarriers for tissue regeneration. J Biomater Appl 33:1242–1251. https://doi.org/10.1177/0885328218825371
Acknowledgements
This work was supported by Zhejiang Provincial Science and Technology Projects (No. LGF18H060007 to YFC), Zhejiang Provincial Natural Science Foundation of China (No. LY17H180010 to XYC), National Natural Science Foundation of China (No. 81672430 to XZM), and Zhejiang Provincial Science and Technology Projects of Traditional Chinese Medicine (No. 2016ZA020 to XYC).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, XY., Chen, JY., Tong, XM. et al. Recent advances in the use of microcarriers for cell cultures and their ex vivo and in vivo applications. Biotechnol Lett 42, 1–10 (2020). https://doi.org/10.1007/s10529-019-02738-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02738-7