Abstract
Objectives
To enhance the yield of 9α-hydroxy-4-androstene-3,17-dione (9-OHAD) from phytosterols, a phytosterol transport system was constructed in Mycobacterium sp. strain MS136.
Results
9-OHAD can be produced via the controlled degradation of phytosterols by mycobacteria. This involves an active transport process that requires trans-membrane proteins and ATP. A phytosterol transport system from Mycobacterium tuberculosis H37Rv was constructed in Mycobacterium sp. strain MS136 by co-expression of an energy-related gene, mceG, and two integrated membrane protein genes, yrbE4A and yrbE4B. The resultant of the Mycobacterium sp. strain MS136-GAB gave 5.7 g 9-OHAD l−1, which was a 20% increase over 4.7 g l−1 by the wild-type strain. The yield of 9-OHAD was increased to 6.0 g l−1 by optimization of fermentation conditions, when 13 g phytosterols l−1 were fermented for 84 h in 30 ml biotransformation medium in shake flasks.
Conclusions
Phytosterol transport system plays an active role in the uptake and transport of sterols, cloning of the system improved the mass transfer of phytosterols and increased the production of 9-OHAD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
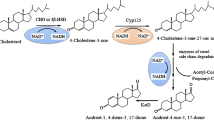
9α-Hydroxy-4-androstene-3,17-dione is the precursor of many steroidal pharmaceuticals, which are widely used as anti-tumor, anti-inflammatory, anti-microbial, anti-viral and anti-fungal drugs (Donova and Egorova 2012). Mycobacteria, Rhodococcus and Nocardia can use sterols as sole carbon and energy sources. The bacteria can then be used for the production of the steroid intermediate, 9-OHAD (Fernandes et al. 2003). The molecular mechanism of the degradation of phytosterol to 9-OHAD can be divided into three steps (Fig. 1). To obtain a high yield of 9-OHAD, previous studies have mainly focused on the modification of the steroidal nuclei, such as the overexpression of 3-steroid-9α-hydroxylation enzyme (KSH) and the knockout of 3-steroid-Δ1-dehydrogenase (KstD) (Knol et al. 2008; Wei et al. 2010), whilst the uptake and transport of phytosterol were relatively rare.
Vancomycin antibiotics, glycine, ethambutol, polyethyleneimine and β-CD can be used to increase the transport of sterol (Wang et al. 2006), but none of these studies involved sterol transport regulated by genetics. With the discovery of microbial sterol-degrading gene clusters (Geize et al. 2007), a Mce4 operon was discovered. This operon system encodes two integrin membrane proteins, which are closely related to the uptake and transport of sterols (Klepp et al. 2012). Rhodococcus jostii RHA1 mutants with deletions of the two integrin membrane proteins’ supAB genes (homologous to yrbE4AB) will lose the ability to grow on cholesterol. In addition, the uptake activity of cholesterol was ATP dependent (Mohn et al. 2008). The mceG gene that encodes transporter ATPases has a significant effect on cholesterol uptake (Joshi et al. 2006; Pandey and Sassetti 2008). Therefore, steroid uptake and transport is an active transport process, which requires transport membrane proteins and ATP.
Steroid mass transfer is the first step in the microbial metabolism, which determines the metabolic flux of the process. Here, we used phytosterols as the substrate, which are slightly different from the cholesterol (Wilbrink et al. 2011), and can be degraded by Mycobacteria. The genes mceG (77%), yrbE4A (90%) and yrbE4B (88%) derived from Mycobacterium tuberculosis H37Rv were highly homologous to the genes of the wild-type strain, and these genes were overexpressed to construct an engineered Mycobacterium strain, enabling a high yield of 9-OHAD.
Materials and methods
Strains, plasmids and reagents
All strains used in this study are listed in Supplementary Table 1. A mixture of phytosterol containing β-sitosterol, campesterol, stigmasterol and brassicasterol (47.5:26.4:17.7:3.6) was purchased from Davi Biochemistry. The standard of 9-OHAD was obtained from Sigma. Other reagents and chemicals used in this study were of the highest grade available.
Culture media and growth conditions
Seed medium was used to cultivate the mycobacterium strains used in this study (10 g glycerol l−1, 1.5 g NH4H2PO4 l−1, 10 g yeast extract l−1, 0.5 g NaH2PO4 l−1, 0.5 g Na2HPO4 l−1, 0.5 g Tween-80 l−1, pH 7.3). Slant medium was used for the passage of the mycobacterium strains. Steroid biotransformation medium were composed of 10 g glycerol l−1, 1.8 g (NH4)2SO4 l−1, 13 g phytosterol l−1, 0.3 g urea l−1, 70 g β-cyclodextrin l−1, 0.8 g NaH2PO4 l−1, 0.5 g Na2HPO4 l−1, and 0.2 g Tween-80 l−1. Escherichia coli DH5a was used as a cloning host for plasmid replication. Kanamycin (50 mg l−1) was supplemented into medium for the selection of mycobacterium transformants. Mycobacteria were cultivated at 30 °C with a shaking speed of 200 rpm.
Plasmid and strain construction
The genes mceG (Genbank ID: 888081), yrbE4A (Genbank ID: 888320) and yrbE4B (Genbank ID: 888336) from Mycobacterium tuberculosis H37Rv were artificially synthesized according to the codon-optimized sequences. The single gene was digested and inserted into the BamHI and HindIII sites of the expression vector pMv261. Then the digested gene segments and expression cassettes were ligated using T4 DNA ligase and transformed into E. coli DH5a. Selected clones were verified by colony PCR and restriction enzyme digestion, while the multiple genes were assembled by overlap extension PCR and then inserted into the expression vector pMv261. In this way, the series of gene expression vectors were constructed (Fig. 2).
Synthetic biology strategies for the construction of the phytosterol transport system in the recombinant Mycobacteria. The transport included one energy-related gene, mceG, and two integrated membrane protein genes, yrbE4A and yrbE4B, from Mycobacterium tuberculosis H37Rv. These gene assemblies were transformed into Mycobacterium sp. strain MS136, respectively, to construct a series of recombinant engineered strains, which were further examined whether the transport of phytosterol could be enhanced
Steroid bioconversion and analysis
The conversion of phytosterols was done in triplicate with cultures grown on 50 ml of transformation medium. After activation of the culture in seed medium for about 48 h at 30 °C and 200 rpm, 10% (v/v) culture broths were inoculated in the conversion reaction system. Bioconversion was monitored for 4 days and sampled every 12 h in flask fermentation at the beginning of the reaction.
The yield was detected by high-performance liquid chromatography (HPLC) method. A sample of the culture was transferred into a clean tube and centrifuged at 4000×g for 15 min. The supernatant was filtered through 0.22 μm pore membrane and then the part of filtrate was injected into the moderate amount of diluent that consists of acetonitrile/water (50:50, v/v). HPLC was performed on a C-18 reserved-phase HPLC column (250 mm × 4.6 mm) under condition at 240 nm with the flow rate 1 ml/min and the mobile phase consisted of acetonitrile/water (520:480) with 0.05% acetic acid. Peaks were compared to an authentic standard of 9-OHAD to determine the exact amount of product during sterol transformation (Supplementary Fig. 1).
Results and discussion
Overexpression of the transport system of phytosterols
A diversity of mceG, yrbE4A and yrbE4B can be usually observed in a few efficient sterol-utilizing strains, indicating its significant role in the sterol uptake. Therefore, we attempted to strengthen the expression of trans-membrane proteins and ATPase activity by overexpressing the sterols transport system in Mycobacterium sp. strain MS-136. The engineered strains could enhance the transformation ability of sterols in different degrees, and the gene mceG had a significant impact on the sterol assimilation. When the gene mceG was co-expressed with yrbE4A and yrbE4B, the yield of 9-OHAD was further improved (Fig. 3). Thus, we obtained a genetically engineered Mycobacterium sp. strain MS136-GAB, with a yield of 5.7 g 9-OHAD l−1, which was a 20% increase over 4.7 g l−1 by the wild-type strain.
Enhancing the production of 9-OHAD by passaging and condition optimization
Many industrial strains need passaging to enhance the productivity. The wild-type Mycobacterium strain was passaged four times to enable a highest yield of 9-OHAD, while the passaging of engineered strains remained unclear. Therefore, we selected the Mycobacterium sp. strain MS136-GAB as the optimal strain to examine the effect of the passage numbers on the production of 9-OHAD. The experiments showed that the engineered strains had the strongest transformation ability in the third generation, which could thus reduce the total fermentation time to a certain extent (Fig. 4a). Excessive substrate could be toxic to the bacteria, we thus varied the concentration of substrate and found that a concentration of 13 g phytosterol l−1 were optimal for the bioconversion reactions to produce 9-OHAD (Fig. 4b).
The 9-OHAD production analyses by different improvements. a The 9-OHAD production differences of Mycobacterium sp. strain MS136 and MS136-GAB under different passages; b effects of system volume on 9-OHAD production by the Mycobacterium sp. strain MS136-GAB; c effects of phytosterol concentrations on 9-OHAD production by the Mycobacterium sp. strain MS136-GAB; d the maximum production of 9-OHAD by genetic engineering and optimization of fermentation conditions. The error bars were calculated from triplicate experiments
Mycobacteria belong to actinomycetes, which are mostly aerobic. The dissolved oxygen level plays a crucial role in bioconversion reactions performed with high substrate concentrations (Gao 2016). In typical shake flask experiments, the oxygen-supply conditions were determined by the system volume. We examined the effect of system volume on the production of 9-OHAD. With the increase in the amount of liquid in the flask, the yield of 9-OHAD was gradually reduced (Fig. 4c). Therefore, 30 ml system was selected as the experimental fermentation system in the subsequent experiments.
Evaluation of the 9-OHAD producer
Considering the importance of phytosterol transport system in Mycobacteria, we engineered the Mycobacterium strain to enhance its phytosterol transport system for increased productivity of 9-OHAD. Initially, we achieved an optimal genetic-engineered strain Mycobacteria sp. strain MS136-GAB by co-expression of the gene mceG and two integrated membrane protein genes, yrbE4A and yrbE4B. The yield of 9-OHAD reached 5.7 g l−1 after three passages, which led to a 20% increase over 4.7 g l−1 by the wild-type strain. Moreover, the yield of 9-OHAD was increased to 6.0 g l−1 by optimization of fermentation conditions (Fig. 4d). When the production of 9-OHAD reached the maximum, the degradation of 9-OHAD also occurred (Fig. 2). According to previous studies, the integrity of 9-OHAD is most at risk from 3-steroid-Δ1-dehydrogenase (KstD), which means the KstD activity may cause the decomposition of 9-OHAD. The inactivation of the KstD gene in Mycobacteria could result in a stable accumulation of 9-OHAD, suggesting the degradation of 9-OHAD is closely related to the expression of the KstD gene (Wei et al. 2010). Thus, deletion of the gene KstD may further improve the productivity of 9-OHAD.
Conclusions
Steroid uptake and transport is an active transport process that requires the assistance of trans-membrane protein and ATP. The gene mceG encoding a transporter ATPase and the two integrin membrane protein genes, yrbE4A and yrbE4B, were identified through the amino acid homology analysis. These genes were heterologously overexpressed to obtain an engineered Mycobacterium sp. strain MS-136-GAB, which could improve the 9-OHAD productivity compared with the wild-type strain. Subsequently, through the passage analysis and the optimization of fermentation conditions, we succeeded in maximizing the yield of 9-OHAD, increasing it from 4.7 to 6.0 g l−1. In conclusion, the important role of the phytosterol transport system for sterol uptake and metabolism was confirmed.
References
Donova MV, Egorova OV (2012) Microbial steroid transformations: current state and prospects. Appl Microbiol Biotechnol 94:1423–1447
Fernandes P, Cruz A, Angelova B, Pinheiro HM, Cabral JMS (2003) Microbial conversion of steroid compounds: recent developments. Enzym Microbial Technol 32:688–705
Gao XQ (2016) Enhanced steroid metabolites production by resting cell phytosterol bioconversion. Chem Biochem Eng Q 29:567–573
Geize RVD, Yam K, Heuser T, Wilbrink MH, Hara H et al (2007) A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc Natl Acad Sci USA 104:1947–1952
Joshi SM, Pandey AK, Capite N, Fortune SM, Rubin EJ, Sassetti CM (2006) Characterization of mycobacterial virulence genes through genetic interaction mapping. Proc Natl Acad Sci USA 103:11760–11765
Klepp LI, Forrellad MA, Osella AV, Blanco FC et al (2012) Impact of the deletion of the six mce operons in Mycobacterium smegmatis. Microbes Infect 14:590–599
Knol J, Bodewits K, Hessels GI, Dijkhuizen L, Geize RVD (2008) 3-Keto-5alpha-steroid delta(1)-dehydrogenase from Rhodococcus erythropolis SQ1 and its orthologue in Mycobacterium tuberculosis H37Rv are highly specific enzymes that function in cholesterol catabolism. Biochem J 410:339–346
Mohn WW, Geize RVD, Stewart GR, Okamoto S, Liu J, Dijkhuizen L, Eltis LD (2008) The actinobacterial mce4 locus encodes a steroid transporter. J Biol Chem 283:35368–35374
Pandey AK, Sassetti CM (2008) Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci USA 105:4376–4380
Wang Z, Zhao F, Chen D, Li D (2006) Biotransformation of phytosterol to produce androsta-diene-dione by resting cells of Mycobacterium in cloud point system. Process Biochem 41:557–561
Wei W, Wang FQ, Fan SY, Wei DZ (2010) Inactivation and augmentation of the primary 3-ketosteroid-Δ1-dehydrogenase in Mycobacterium neoaurum NwIB-01: biotransformation of soybean phytosterols to 4-androstene-3,17-dione or 1,4-androstadiene-3,17-dione. Appl Environ Microbiol 76:4578–4582
Wilbrink MH, Petrsma M, Dijkhuizen L, Geize RVD (2011) FadD19 of Rhodococcus rhodochrous DSM43269, a steroid-coenzyme a ligase essential for degradation of C-24 branched sterol side chains. Appl Environ Microbiol 77:4455–4464
Acknowledgements
The authors acknowledge the financial support by a startup funding from Tianjin University.
Supporting information
Supplementary Table 1—Strains, primers and plasmids used in this study.
Supplementary Fig. 1—HPLC analysis of 9-OHAD produced by engineered strains.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, K., Sun, H. & Song, H. Engineering phytosterol transport system in Mycobacterium sp. strain MS136 enhances production of 9α-hydroxy-4-androstene-3,17-dione. Biotechnol Lett 40, 673–678 (2018). https://doi.org/10.1007/s10529-018-2520-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-018-2520-9