Abstract
Objectives
To optimize the expression of type A ferulic acid esterase (FaeA) from Aspergillus niger in Pichia pastoris X-33 using codon optimization.
Results
Recombinant FaeA was purified from the fermentation broth, with the maximum specific activity of 48.4 ± 0.1 U mg−1. Adding it during mashing process for beer brewing raised the filtration rate by 14.5% while the turbidity and viscosity declined by 22 and 6.9%, respectively. Addition of FaeA increased the concentrations of free ferulic acid (FA) and arabinoxylan (AX) in the wort, while the polymeric arabinoxylans content declined significantly.
Conclusions
Recombinant FaeA was capable to prevent the oxidative gelation of PAX formation by breaking the cross-linking of FA among AX chains and improve the filtration performance of wort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ferulic acid (FA) is the most abundant phenolic acid in barley and malt. It is concentrated mainly in the aleurone layer, husk, and the endosperm in free and bound as well as monomer and oligomer forms (Humberstone and Briggs 2015). FA is esterified to the C5-hydroxyl group of R-L-arabinofuranosyl subunits of the xylan backbone to form arabinoxylan (AX), which is a primary constituent of the aleurone layer (about 85%) and starchy endosperm (about 23%) in barley and malt (Humberstone and Briggs 2015). AX plays an important role in wort filtration and determines the quality of the final beer. The cross-linking of FA among AX chains, catalyzed by a peroxidase in the wort, adversely affects wort filterability (Li et al. 2005). With the formation of water-extractable arabinoxylan (WEAX) gel, the filtration rate of wort decreases significantly, while both turbidity and viscosity of the wort increase (Wu et al. 2016). The lack of hydrolytic enzymes, such as ferulic acid esterase (FAE), is an important reason why most AX remain in the malt, which leads to poor wort filtration. Therefore, AX-induced filtration problems could be solved by adding FAE during the mashing process.
Ferulic acid esterase (E.C 3.1.1.73) is one of the subtypes of carboxylic ester hydrolase, which can degrade the ester linkage between polysaccharide and FA in plant cell walls, resulting in the release of free monomer or dimer FA. It is classified into four types (Types A–D) based on the primary structure and substrate specificity toward substrate aromatic moieties (Crepin et al. 2004). More than 30 types of FAE from different species, including Aspergillus niger, A. oryzae, Streptomyces olitrochromogenes, and Saccharomyces cerevisiae have been separated (Gopalan et al. 2015). However, the low yield of FAE, usually below 1 U ml−1 culture medium, for most wild-type strains limits its industrial applications (Gopalan et al. 2015). FAE production has now shifted toward heterologous expression mainly using two hosts, Escherichia coli and Pichia pastoris. The gene coding for FaeA from A. niger has been heterologous expressed in P. pastoris and the yield of recombinant FaeA was 300 mg l−1 (Juge et al. 2001). As P. pastoris has its own preferred codons, the exogenous gene containing non-preferred codons could suppress its expression.
In this study, codon optimization was used to enhance the heterologous expression of Anfae A (encoding FaeA in A. niger) in P. pastoris under the premise of retaining the composition of amino acids. Finally, the recombinant FaeA was purified and added during mashing process to degrade the FA cross-linking between AX chains and consequently improve the filtration performance of wort.
Materials and methods
Strains and plasmids
Aspergillus niger CBS 513.88, used for amplifying Anfae A, was cultured on potato/dextrose/agar (PDA) medium (24 g l−1 potato/dextrose broth and 20 g l−1 agar) at 37 °C. Pichia pastoris X-33, used for heterologous expression of FaeA, was grown in yeast extract/peptone/dextrose (YPD) medium (10 g l−1 yeast extract, 20 g l−1 peptone, and 20 g l−1 glucose) at 30 °C. Plasmid pPICZαA containing methanol-inducible alcohol oxidase 1 (AOX1) promoter that used for expression Anfae A was purchased from TaKaRa. Screening medium was prepared by adding 1.5 ml dimethylformamide, containing 10% (v/v) ethyl ferulate (Sigma), into 100 ml sterilized 2% (w/v) agar. Commercial Dan’er malt, with a slow wort filtration rate and high turbidity, was provided by a commercial malting company in the Jiangsu Province of China.
Gene cloning of Anfae A from A. niger
Signal P3.0 was used to predict the sequence signal peptide of Anfae A and design primers for overlap PCR according to the sequences reported by NCBI (GenBank: Y09330.2). The genome of A. niger was isolated by the CTAB method and used for PCR amplification as a template. Extron 1 and extron 2 were amplified by primer pairs F1/R1 and F2/R2 (Supplementary Table 1), respectively. The products were purified and pooled with an equimolar amount as a template for overlap PCR using primer pair F1/R2 to obtain Anfae A gene that without signal peptides and introns. The amplified fragment was purified and then sequenced (Sangon).
Codon optimization and sequence synthesis
Two strategies, including “random optimization” (http://www.jcat.de/) and “one by one optimization” (Hu et al. 2006), were used to optimize the preferred codons of Anfae A from P. pastoris. The first strategy consisted of randomly assigning a triplet for each amino acid according to the codons in Supplementary Table 2, with a probability based on the weight of each codon within the set encoding a given amino acid (Menzella 2011). In “one by one optimization” method, the preferred codons in the genome of P. pastoris were assigned to each amino acid (Supplementary Table 2).
Construction and transformation of recombinant plasmids
The initially amplified Anfae A and two optimized fragments were double digested with EcoR I and Not I and then ligated with plasmid pPICZαA, respectively, which was treated with the same restriction endonucleases, to generate recombinant plasmids. The constructed plasmids were linearized with Sac I and transformed, respectively into P. pastoris X-33 according to the protocol of Pichia Transformation Kit (Thermo Fisher). Transformants were plated on the selective YPD plates and cultured at 30 °C until strains were ready to pick. The genome of the colony strain was isolated and used as a template for PCR identification.
Heterologous expression and purification of the recombinant ferulic acid esterase
15 ml of the overnight culture was inoculated in 500 ml fresh YPD medium, containing 200 μg ml−1 Zeocin, and induced by 0.1% methanol at 30 °C 220 rpm for 108 h. The recombinant FaeA was firstly purified by 1 ml affinity chromatography His-trap (GE) according to its operation instruction. Briefly, the fermentation broth was centrifuged and filtered via 0.22 μm aquo-system membrane (Sangon). 10 ml samples were loaded onto the equilibrated column with the flow rate of 0.5 ml min−1. After washing with 20 column volume of wash buffer (20 mM sodium phosphate, 0.5 M NaCl, 100 mM imidazole, pH 6.8), 10 column volume of elution buffer (20 mM sodium phosphate, 0.5 M NaCl, 500 mM imidazole, pH 6.8) was used for one-step procedure, with flow rate of 1 ml min−1. The fraction with FaeA activity was determined and analyzed using SDS-PAGE. The eluent that merely containing FaeA was collected and dialyzed with a dialysis bag (8–14 kDa, Sinopharrn) to remove imidazole. The purified FaeA was concentrated by ultrafiltration (Model: Amicon Ultra-15, 10 kDa, Millipore) and stored at 4 °C for further use after lyophilization.
Activity assay of recombinant ferulic acid esterase
The activity of FaeA was determined according to the previously described method (Mastihuba et al. 2002). One unit (U) of enzymatic activity is defined as the amount of enzyme required to release 1 μmol of FA per minute under the assay conditions.
Addition of recombinant ferulic acid esterase during mashing process
Standard European Brewery Convention (EBC) congress mashing was conducted in a temperature-controlled mash bath (Model: LB-12, Lochner Labor) using 50 g Dan’er malt. A series amount of FaeA (0, 0.104, 0.416, and 1.04 U) was respectively added into the mash at the beginning. Furthermore, at the end of the saccharification process, the filtration rate, and viscosity were measured via EBC official analytical methods to evaluate the filterability of congress wort. Moreover, the content of AX was determined using gas chromatography (Li et al. 2005) and the concentration of PAX was monitored using the same method after precipitation by 80% ethanol.
Statistical analysis
The data were obtained from three biological replicate trials of each experiment and analyzed with SPSS 19.0. One-way analysis of variance was used to analyze the data at the 95% confidence level (p < 0.05).
Results
Cloning the original Anfae A gene and synthesis of the optimized genes
A 780-bp fragment that contained extron 1 and extron 2 from A. niger was obtained via overlap PCR. The sequencing results suggested that the amplified fragment showed 99.64% identity with the sequence of Anfae A in NCBI (GenBank: Y09330.2), indicating that the functional Anfae A was successfully cloned. In comparison with online analysis (http://www.kazusa.or.jp/codon) of synonymous codon usage frequency of P. pastoris (Supplementary Table 2), some amino acid residues in Anfae A were encoded by codons that were rarely present in the host, such as CCG (Pro), GCG (Ala), CTC (Leu), TCG/AGC (Ser), all of which had less than 15% of usage . Moreover, more than half of these rare codons usually exist in clusters of 2–6 consecutive codons, resulting in a much lower expression in P. pastoris. Thus, the codon optimization of Anfae A gene was performed using “random optimization” and “one by one optimization” strategies, respectively, to increase the protein expression.
The sequences of original Anfae A gene and that after optimization were named Anfae A-ori, Anfae A-opt I, and Anfae A-opt II, respectively. As shown in Supplementary Fig. 1, the GC content in Anfae A-ori sequence was 54.4%, which respectively declined to be 45.1 and 40.9% in Anfae A-opt I and Anfae A-opt II. In addition, the codon adaptation index (CAI) can be used to assess the adaptation of viral genes to their hosts and make comparison of codon usage in different organisms (Fuglsang 2003). In fact, compared with Anfae A-ori, the CAI values in Anfae A-opt I and Anfae A-opt II were enhanced from 0.51 to 0.91 and 0.97, respectively.
Construction of the expression plasmid and screening of positive transformants
Three gene fragments with EcoRI and NotI sites at two ends were amplified and double digested, which were then respectively ligated with plasmid pPICZαA to obtain the recombinant plasmids, pPICZαA-Anfae A, pPICZαA-Anfae A-opt I, and pPICZαA-Anfae A-opt II. The results of colony PCR and sequencing indicated that the three expression plasmids were successfully constructed. After linearization by Sac I, the three plasmids were respectively transformed into P. pastoris X-33, via homologous recombination at the AOX1 promoter. The positive tranformants picked up from the selective YPD medium were verified via both PCR and sequencing. Moreover, one transformant of each type with highest enzyme activity of FaeA was selected according to the transparent circle diameter (Supplementary Fig. 2) and named FaeA-ori, FaeA-opt I, and FaeA-opt II, respectively.
Purification of the recombinant ferulic acid esterase
FaeA activities in different cultures with FaeA-ori, FaeA-opt I and FaeA-opt II were measured and the results were shown in Fig. 1. The activity of FaeA in the fermentation of FaeA-opt I was 39.9 U ml−1, which was about sixfold higher than that of FaeA-ori (6.8 U ml−1). While the FaeA-opt II strain displayed approximately 76.5% FaeA activity compared to FaeA-ori. These results indicated that the “random optimization” strategy could efficiently improve FaeA expression in P. pastoris. In contrast, the FaeA activity declined after “one by one optimization.” The possible reason was that rare codons were replaced by high-frequency codons in the “one by one optimization” method, but the distribution of GC bases were not optimized. Actually, gene expression was influenced by the GC content and its distribution, while the “random optimization” strategy could consider the above factors, leading to significant improvement of FaeA expression.
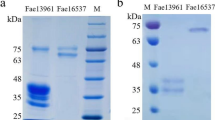
As shown in Supplementary Table 3, the concentrations of heterogeneous protein in the supernatant produced by FaeA-ori, FaeA-opt I, and FaeA-opt II were 150 ± 4, 858 ± 8, and 118 ± 4 mg l−1, respectively, while the specific activity of FaeA were 45.7 ± 0.3, 46.5 ± 0.2, and 44.2 ± 0.3 U mg−1. The results indicated that the expression amount rather than the specific activity of FaeA was significantly increased after codon optimization. Since His-tag was present at the N-terminal, the recombinant FaeA was easily purified from the fermentation of FaeA-opt I by Ni–NTA affinity chromatography. The results of SDS-PAGE showed a single band at 33 kDa (Fig. 2), the molecular weight of which was similar to crude protein. The specific activity of the purified recombinant FaeA was 48.4 ± 0.1 U mg−1.
The positive effect of recombinant FaeA on wort filterability
To confirm the recombinant FaeA was capable to improve the filtration performance of wort, a series of FaeA amounts (0, 0.104, 0.416, and 1.04 U) was added at the beginning of mashing process using Dan’er malt. This showed that with increased FaeA addition, the filterability of wort improved (Fig. 3). Compared to the initial wort (the addition of FaeA was 0), the filtration rate increased by 14.5%, the turbidity and viscosity, respectively declined by 22 and 6.9% at the addition level of 1.0 U 50 g malt−1. The results suggested that the high concentration or activity of FaeA was the promoting factor of wort filterability. Furthermore, the contents of FA, AX, and PAX in four wort with different FaeA addition were monitored (Table 1). More FaeA addition dramatically increased the concentrations of FA and AX, while the content of PAX significantly declined. The results indicated that the added FaeA was capable to break FA cross-linking between AX chains and subsequently prevent the oxidative gelation to form PAX, resulting in the release of free FA and AX.
Conclusion
Three types of FAE have been discovered (FaeA, FaeB, and FaeC) in A. niger (de Vries et al. 2002, 1997; Dilokpimol et al. 2017), the first two of which are currently used in many industrial production processes. In the current study, we expressed the Anfae A gene (encoding FaeA) from A. niger CBS 513.88 in P. pastoris X-33 and applied this to the mashing process of Dan’er malt to improve its filtration performance. Firstly, the sequence of the Anfae A gene was optimized via two strategies according to the codon preference of P. pastoris. The expression amount of FaeA produced by FaeA-opt I was enhanced and its activity was approximately sixfold higher than that of the original one after “random optimization,” which coincided with previous research (Chen et al. 2016). With more recombinant FaeA added during mashing process, FA concentrations and the filtration rate of wort increased. This indicated that oxidative gelation between WEAX could be degraded by FaeA, this improving the filtration performance. Moreover, as a strong antioxidant, FA is effective at reducing the formation of carbonyl compounds in beer (Walters et al. 1997). Overall, the addition of recombinant FaeA is likely very beneficial for the quality traits of wort and the final beer.
References
Chen X, Zhou M, Huang Z, Jia G, Liu G, Zhao H (2016) Codon optimization of Aspergillus niger feruloyl esterase and its expression in Pichia pastoris. Biologia 71:626–631
Crepin V, Faulds C, Connerton I (2004) Functional classification of the microbial feruloyl esterases. Appl Microbiol Biotechnol 63:647–652
de Vries R, Michelsen B, Poulsen C, Kroon P, van Den Heuvel R, Faulds C, Williamson G, van Den Hombergh J, Visser J (1997) The faeA genes from Aspergillus niger and Aspergillus tubingensis encode ferulic acid esterases involved in degradation of complex cell wall polysaccharides. Appl Environ Microbiol 63:4638–4644
de Vries R, Kester HC, Visser J (2002) The A. niger faeB gene encodes a second feruloyl esterase involved in pectin and xylan degradation and is specifically induced in the presence of aromatic compounds. Biochem J 363:377–386
Dilokpimol A, Mäkelä MR, Mansouri S, Belova O, Waterstraat M, Bunzel M, de Vries RP, Hildén KS (2017) Expanding the feruloyl esterase gene family of Aspergillus niger by characterization of a feruloyl esterase, FaeC. New Biotechnol 37:200–209
Fuglsang A (2003) Codon optimizer: a freeware tool for codon optimization. Protein Expres Purif 31:247–249
Gopalan N, Rodríguez L, Saucedo G, Nampoothiri KM (2015) Review on technological and scientific aspects of feruloyl esterases: a versatile enzyme for biorefining of biomass. Bioresour Technol 193:534–544
Hu S, Li L, Qiao J, Guo Y, Cheng L, Liu J (2006) Codon optimization, expression, and characterization of an internalizing anti-ErbB2 single-chain antibody in Pichia pastoris. Protein Expres Purif 47:249–257
Humberstone FJ, Briggs DE (2015) Partial purification of ferulic acid esterase from malted barley. J Inst Brew 108:439–443
Juge N, Williamson G, Puigserver A, Cummings NJ, Connerton IF, Faulds CB (2001) High-level production of recombinant A. niger cinnamoyl esterase (FAEA) in the methylotrophic yeast Pichia pastoris. FEMS Yeast Res 1:127–132
Li Y, Lu J, Gu GX, Shi ZP, Mao ZG (2005) Studies on water-extractable arabinoxylans during malting and brewing. Food Chem 93:33–38
Mastihuba VR, Kremnický LR, Mastihubová M, Willett JL, Côté GL (2002) A spectrophotometric assay for feruloyl esterases. Anal Biochem 309:96–101
Menzella HG (2011) Comparison of two codon optimization strategies to enhance recombinant protein production in Escherichia coli. Microb Cell Fact 10:1–8
Walters MT, Heasman AP, Hughes PS (1997) Comparison of (+)-catechin and ferulic acid as natural antioxidants and their impact on beer flavor stability. 1. Forced-aging. J Am Soc Brew Chem 55:83–89
Wu DH, Zhou T, Li XM, Cai GL, Lu J (2016) POD promoted oxidative gelation of water-extractable arabinoxylan through ferulic acid dimers. Evidence for its negative effect on malt filterability. Food Chem 197:422–426
Acknowledgements
This work was supported by the National High Technology Research and Development Program of China (2013AA102109), the National Natural Science Foundation of China (31701588), the Natural Science Foundation of Jiangsu Province, China (BK20170178), the Project Funded by the Program of Introducing Talents of Discipline to Universities (111 Project) (111-2-06), the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Collaborative Innovation Center of Jiangsu Modern Industrial Fermentation.
Supporting information
Supplementary Table 1—Primers used in this study.
Supplementary Table 2—Optimal codons in Pichia pastoris.
Supplementary Table 3—Comparison of the expression and activity levels of FaeA in different strains.
Supplementary Figure 1—Alignment of nucleotide of the optimized and original Anfae A genes. Optimized sequences were designed using the GeMS software package and then synthesized by Sangon. Characters with shadow were the same nucleotides, and others were different.
Supplementary Figure 2—Screening of positive transformants with high expression of recombinant FaeA. The colonies were firstly cultured in buffered glycerol complex medium (1% yeast extract, 2% tryptone, 1.34% YNB, 1% glycerol, 4×10−5% biotin, and 100 mmol l-1 potassium phosphate, pH 6.0) with shaking at 30°C 220 rpm to reach OD600 2-6. The cells were then harvested via centrifugation and suspended in buffered methanol-complex medium (1% yeast extract, 2% tryptone, 1.34% YNB, 4×10−5% biotin, 1% methanol, and 100 mmol l-1 potassium phosphate, pH 6.0). After further culture for 96 h, 1% methanol was added every 24 h to induce the expression of recombinant FaeA. The supernatant of fermentation was added into the ethyl ferulate plates and positive transformants with highest activity of FaeA were selected according to the transparent circle diameter. The larger diameter of the transparent circle indicated higher FaeA activity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10529_2018_2511_MOESM1_ESM.tif
Supplementary material 1 (TIFF 14051 kb). Supplementary Fig. 1—— Alignment of nucleotide of the optimized and original Anfae A genes. Optimized sequences were designed using the GeMS software package and then synthesized by Sangon. Characters with shadow were the same nucleotides, and others were different
10529_2018_2511_MOESM2_ESM.tif
Supplementary material 2 (TIFF 15745 kb). Supplementary Fig. 2——Screening of positive transformants with high expression of recombinant FaeA. The colonies were firstly cultured in buffered glycerol complex medium (1% yeast extract, 2% tryptone, 1.34% YNB, 1% glycerol, 4 × 10−5 % biotin, and 100 mmol l−1 potassium phosphate, pH 6.0) with shaking at 30 °C 220 rpm to reach OD600 2-6. The cells were then harvested via centrifugation and suspended in a buffered methanol-complex medium (1% yeast extract, 2% tryptone, 1.34% YNB, 4 × 10−5 % biotin, 1% methanol, and 100 mmol l−1 potassium phosphate, pH 6.0). After further culture for 96 h, 1% methanol was added every 24 h to induce the expression of recombinant FaeA. The supernatant of fermentation was added into the ethyl ferulate plates and positive transformants with the highest activity of FaeA were selected according to the transparent circle diameter. The larger diameter of the transparent circle indicated higher FaeA activity
Rights and permissions
About this article
Cite this article
Wu, D., Cai, G., Li, X. et al. Cloning and expression of ferulic acid esterase gene and its effect on wort filterability. Biotechnol Lett 40, 711–717 (2018). https://doi.org/10.1007/s10529-018-2511-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-018-2511-x