Abstract
Objective
To isolate a thermostable pyrimidine nucleoside phosphorylase (PyNP) from mesophilic bacteria by gene mining.
Results
BbPyNP from Brevibacillus borstelensis LK01 was isolated by gene mining. BbPyNP had a highest 60% identity with that of reported PyNPs. BbPyNP could catalyze the phosphorolysis of thymidine, 2′-deoxyuridine, uridine and 5-methyuridine. BbPyNP had good thermostability and retained 73% of its original activity after 2 h incubation at 50 °C. BbPyNP had the highest activity at an optimum alkaline pH of 8.5. BbPyNP was stable from pH 7 to 9.8. Under preliminary optimized conditions, the biosynthesis of various 5-halogenated pyrimidine nucleosides by BbPyNP reached the yield of 61–84%.
Conclusion
An efficient approach was estimated in isolating thermostable PyNP from mesophilic bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Structural nucleoside analogues (NAs) are widely used in cancer and viral infection (Mikhailopulo and Miroshnikov 2010). Many NAs, including pyrimidine and purine NAs, have been applied in clinical therapies. For example, 5-fluorouracil is of major value in treating solid cancers, such as breast, ovary, and gastrointestinal cancers (Grem 2000; Liu et al. 2009). In addition, 2′-deoxy-5-fluorouridine is useful for the design of new prodrugs. Halogenated nucleosides among the important nucleoside analogs show improved properties of pharmacological activity and have been explored as potential anti-cancer and antivirus agents (Liu et al. 2009).
The majority of NAs are traditionally prepared by various chemical methods, which employ several steps including protection and deprotection procedures because of the low region and stereoselectivity. Compared with harsh experimental condition employed by chemical methods, enzymatic synthesis of natural or unnatural nucleosides exhibits many attractive features, such as the reaction can be performed under mild conditions and thus tedious protection/deprotection steps in selective modification of nucleoside derivatives are avoided (Qian et al. 2008). Indeed, only β-anomers are produced by the enzymatic synthesis of unnatural nucleosides. The nucleoside phosphorylases can catalyze reversible phosphorolytic cleavage of N-glycosidic bonds of nucleosides in the presence of inorganic phosphate ions. Thus, NAs can be synthesized when the intermediates couple to other modified bases. Cells of eukaryotes and some types of prokaryotes contain two specific pyrimidine nucleoside phosphorylases (PyNPs), including thymidine nucleoside phosphorylase (EC 2.4.2.4) and uridine phosphorylase (EC 2.4.2.3). In some types of prokaryotes, non-specific PyNP (EC 2.4.2.2) serves as an alternative to the above mentioned enzymes. The latter enzyme catalyzes the phosphorolysis of uridine and thymidine with equal catalytic efficiency (Balaev et al. 2016). Until now, study on thermostable PyNPs from thermophiles for efficient biosynthesis of NAs is approved as high temperature could promote solubility of nucleosides. The majority of thermostable PyNPs were from mesophilic bacteria. To fully develop microbial and enzyme resources, diverse PyNPs would be appreciated for expanding the synthesis of various NAs. Thus, the screening of PyNP from mesophilic strains would be significant for efficient enzymes.
In this study, a PyNP with good thermostability and broad range of alkaline pH tolerance from mesophilic bacteria was obtained by gene mining. Several 5-halogenated pyrimidine nucleosides were successfully synthesized by BbPyNP.
Materials and methods
Materials
Nucleosides and their bases were purchased from Aladdin (Shanghai, China), and other chemicals were of analytical grade and purchased from Sunshine (Nanjing, China). Solvents used in high-performance liquid chromatography (HPLC) were of HPLC grade. One Step Cloning Kit was purchased from Novoprotein (Shanghai, China).
Gene mining of PyNPs
GsPyNP (GenBank: BAA13512.1) from Geobacillus stearothermophilus was selected as a template to mine thermostable PyNPs. GsPyNP has been employed in the synthesis of modified nucleosides at high temperature with a high yield (Taran et al. 2009). By using the amide acid sequence of GsPyNP as contrast, PyNPs from mesophilic bacteria over a broad range of identities were analyzed. AmPyNP (GenBank: WP 043069198.1) from Aneurinibacillus migulanus, BfPyNP (GenBank: WP 018706450) from Bacillus fordii, BbPyNP (GenBank: EMT53684) Brevibacillus borstelensis, LpPyNP (GenBank: WP 057708581) from Lactobacillus paracasei, and SmPyNP (GenBank: ALE99123) from Serratia marcencens were selected as potential PyNPs by amino acid sequence alignment for further investigation.
Candidate gene clone, expression and purification
Targeted strains were grown overnight on lysogeny broth/agar plates or in LB at 37 °C. The genomic DNA was extracted from broken cells using AP-MN-BT-GDNA-50 (Axygen BioScience, Inc.). The genes coding for PyNP from mesophilic strains preserved in our lab, Aneurinibacillus migulanus U603, Bacillus fordii, Brevibacillus borstelensis LK01, Lactobacillus paracasei A1, and Serratia marcencens MH6 were amplified using EVO DNA polymerase (Takara, Japan). The primers are listed in Supplementary Table 1. The vector pET-28a (Invitrogen, USA) with kanamycin resistance was used for enzyme expression. The recombinant plasmids were constructed using ClonExpress II One Step Cloning Kit, and transformed to Escherichia coli BL21(DE3) (Novagen) respectively.
Escherichia coli BL21(DE3) strains with recombinant pPyNPs were grown overnight in LB containing kanamycin. The expression of PyNPs was conducted by addition of 1 mM IPTG when the OD600 reached 0.6–0.8. The cells were harvested by centrifugation (12,000×g, 4 °C, 10 min) after induction for 8 h. The cell pellets were suspended in PBS (Na2HPO4/KH2PO4, 1/15 M, pH 8.0). The cell suspension was subjected to ultrasonic fragmentation on ice by using a GA92-IID ultrasonicator, and centrifuged at 12,000×g for 10 min (4 °C). Then the supernatant was applied to Ni–NTA Superflow cartridge (Qiagen). The excess of imidazole in the eluent was removed by dialysis against 50 mM PBS (pH 8.0) at 4 °C. The purity of PyNP was analyzed by SDS-PAGE electrophoresis (4–12%).
Enzyme activity assay
The enzyme activity assay was performed in a reaction mixture (pH 8.5, 50 mM Na2HPO4/KH2PO4) containing 1 mM EDTA and 10 mM uridine as substrate. After the substrate was preheated for 5 min, 50 μL purified enzyme was added to 1 mL reaction mixture, and the reaction was performed at 50 °C for 10 min. A total of 50 μL of reaction mixture was transferred to 950 μL methanol to stop the reaction,and then the uracil produced from uridine by PyNP was analyzed by HPLC (Hori et al. 1990). One unit of enzyme activity (U) was defined as the amount of the enzyme required to liberate 1 μmoL uracil per min.
Effect of pH on activity and stability
The pH profile of the PyNP was determined using 50 mM Na2HPO4/KH2PO4 buffers at pH 4.9–9.2, and NaOH/KH2PO4 buffers at pH 8.6–10.6. Residual activity after 2 h of incubation at various pH values was determined to test pH stability.
Effect of temperature on activity and thermostability
The temperature profile of PyNP was determined in 50 mM PBS (pH 8.5) from 40 to 80 °C over 10 min. To evaluate the thermostability of PyNP, the purified enzyme was diluted appropriately and then incubated in thin-wall microtubes from 40 to 65 °C. Subsequently, the residual activity was determined and calculated by setting the initial activity as 100%.
Kinetic analysis of BbPyNP
The kinetic parameters were determined in the reaction mixture containing nucleosides as substrates. Initial velocities of the phosphorolysis reaction were determined with substrate from 0.2 to 5 mM. The apparent Km values for substrates in the presence of a saturating concentration of the counter substrate were determined by fitting the initial velocity data to the Michaelis–Menten equation using non-linear regression analysis.
Biosynthesis of nucleoside analogues (NAs)
The purified BbPyNP was applied to catalyze the phosphorolysis of different pyrimidine nucleosides. The biosynthesis of 5-fluoro-2′-deoxyuridine (FDUR) was tested as a model to optimize the process. Key reaction parameters such as pH, temperature, and molar ratio, were preliminarily optimized, and other halogenated nucleosides were synthesized under optimized conditions.
Quantitative analysis of halogenated nucleosides
The reaction mixture was analyzed by HPLC equipped with Agilent TC-C18 column (4.6 mm × 250 mm, 5 μm) at 30 °C. The mobile phase was water/methanol (95/5, v/v) at 1 mL min−1. Biosynthesis of the product was further confirmed by MALDI-TOF-MS, and NMR spectroscopy (Bruker AV-300).
Results and discussion
Screening of thermostable PyNP by gene mining
Mesophilic strains may produce thermally stable enzymes. It is thus possible to achieve PyNP with good thermostability from mesophilic bacteria. Gene mining of potential PyNPs by using GsPyNP (GenBank: BAA13512.1) from G. stearothermophilus as a template could provide the advantage of efficient biocatalysis for NAs synthesis. To fully develop microbial resources, mesophilic bacteria were isolated and preserved in our lab. Possible PyNPs from those mesophilic bacteria were analyzed. The sequence alignment of five potential PyNPs was basically consistent with the putative PyNPs (Table 1). The possible PyNPs showed 38.9–76.8% amino acid identities to some reported PyNPs with good thermostability including GsPyNP, BsPyNP, TtPyNP and GtPyNP (Taran et al. 2009; Serra et al. 2013; Szeker et al. 2012, 2012). AmPyNP from A. migulanus, BfPyNP from B. fordii, BbPyNP from B. borstelensis, LpPyNP from L. paracasei, and SmPyNP from S. marcencens showed the relatively high identities of 76.8, 68.8, 60, 64.6, and 42% with the corresponding reported PyNPs respectively.
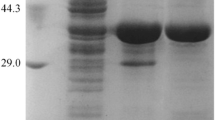
The five selected PyNPs from the mesophilic strains preserved in our laboratory were cloned and expressed in E. coli respectively. AmPyNP from A. migulanus U603, BfPyNP from B. fordii, BbPyNP (Genbank: KY031982) from B. borstelensis LK01, LpPyNP from L. paracasei A1, and SmPyNP from S. marcencens MH6 was isolated respectively. The five above-mentioned PyNPs with His-tagged were expressed and then purified by Ni2+-affinity chromatography. Purified recombinant BbPyNP was obtained (Fig. 1), and other recombinant PyNPs were also purified (data not shown). Thermal stability and special activity toward uridine were tested using purified PyNPs. The special activity and thermostability of five isolated PyNPs are investigated (Table 2). Obviously, BbPyNP had higher special phosphorylase activity toward uridine than other obtained PyNPs. Moreover, BbPyNP exhibited the best thermostability of five purified PyNPs. Thus, BbPyNP was chosen as potential biocatalyst.
Substrate specificity of BbPyNP
As shown in Table 3, BbPyNP had phosphorolysis activity toward deoxyuridine, thymidine, uridine, and 5-methyluridine but not arauridine, 2′,3′-deoxyuridine, cytidine, or deoxycytidine. It was found that cytidine cannot be cleavaged by the nucleoside phosphorylase. The PyNPs from Bacillus subtilis (Serra et al. 2013), Bacillus stearothermophilus JTS859 (Hori et al. 1990), and B. stearothermophilus TH 6-2 (Okuyama et al. 1996) also showed maximal phosphorolysis of deoxyuridine. These PyNPs also had the same substrate specificity. As shown in Table 4, the kinetic parameter of Km indicated that BbPyNP had the higher affinity to deoxyuridine than that of other substrates. Interestingly, K m of BbPyNP to thymidine was similar to that of thermostable PyNP from Geobacillus thermoglucosidasius (Szeker et al. 2012). Both of the enzymes had higher K m to uridine than that to thymidine.
Effect of pH on activity and stability of BbPyNP
As illustrated in Fig. 2a, BbPyNP had an approx. optimal pH of 8.5, and showed more than 85% maximal activity from pH 7.4 to 9.2. BbPyNP had higher activity in the alkaline buffer than in the acidic buffer. As illustrated in Fig. 2b, BbPyNP was stable in alkaline buffer and retained more than 90% of its original activity after 2 h incubation from pH 7 to 9.8. The alkaline buffer could increase the nucleoside concentration. Higher yields were also achieved in a system with alkaline pH 10 than in a system with pH 7 by the biosynthesis of 2′-deoxynucleosides with nucleoside phosphorylases from B. subtilis (Serra et al. 2013).
Characteristics of the purified BbPyNP. a Effects of pH on activity of BbPyNP. The assays were performed at 37 °C in 50 mM Na2HPO4/K2HPO4 or NaOH/KH2PO4 buffers. b Effects of pH on stability of BbPyNP. Samples were pre-incubated for 2 h at 40 °C before measuring enzyme activity. c Effects of temperature on activity of BbPyNP. The assays were performed at pH 8.5 in 50 mM Na2HPO4/K2HPO4 buffers. d Thermal stability of BbPyNP. After pre-incubating for few hours at different temperatures, the activities of samples were measured. The enzyme activity was measured at pH 8.5 and 50 °C in 50 mM phosphate buffer. Each experiment was performed in triplicate
Effect of temperature on activity and stability of BbPyNP
As shown in Fig. 2c, the optimum temperature of BbPyNP for phosphorolysis activity was 60 °C which is similar to that of thermostable PyNP from G. thermoglucosidasius (Szeker et al. 2012). As shown in Fig. 2d, BbPyNP retained 73% of its initial activity after 2 h of at 50 °C. The half-life of BbPyNP was 3.4 h at 55 °C (data not shown). The half-life of GtPyNP from G. thermogluosidasius was 1.6 h at 70 °C, with no loss of activity within 16 h at 60 °C (Szeker et al. 2012). The half-life of PyNP from Thermus thermophilus was more than 24 h at 80 °C (Szeker et al. 2012). The half-life of PyNP from G. stearothermophilus JTS859 was 25 min at 70 °C (Hori et al. 1990). The BbPyNP showed a tolerance to relatively high temperature. This may be beneficial to promote the solubility of substrate. This result suggested that BbPyNP from mesophilic bacteria B. borstelensis LK01 was a relatively thermostable enzyme, and BbPyNP could be used for the biosynthesis of NAs.
Biosynthesis of 5-halogenated nucleosides with BbPyNP
With the aim of investigating of biosynthesis, 5-fluouracil was selected as model substrate to optimize the conversion. The efficient synthesis of FDUR was achieved with the conversion of 77% at 50 °C, pH 8 using 50 mM 2′-deoxyuridine and 10 mM 5-fluorouracil as substrate. The product showed main ion peak at m/z 245.059 [M-H]− in a negative TOF-MS-ES spectrum, corresponding to FDUR (Supplementary Fig. 1). 1H- and 13C-NMR spectra of 5-fluoro-2′-deoxyuridine (FDUR) were recorded using a Bruker AV-300 spectrometer with tetramethylsilane (TMS) as internal standard. 1H-NMR of 5-fluoro-2′-deoxyuridine (300 MHz, DMSO-d6) δ: 6.13 (1H, H-1′), 2.50 (1H, H-2′), 4.24 (1H, H-3′), 3.78 (1H, H-4′), 3.59 (2H, H-5′), 8.17 (1H, H-6). 13C-NMR of 5-fluoro-2′-deoxyuridine (75 MHz, DMSO-d6) δ: 84.51 (C-1′), 40.33 (C-2′), 70.11(C-3′), 87.43(C-4′), 61.01 (C-5′), 149.48 (C-2), 157.79 (C-4), 138.52 (C-5), 124.21(C-6) (Supplementary Figs. 2, 3). The MS and NMR spectroscopic characterization of the product was identical to that of authentic 5-fluoro-2′-deoxyuridine (FDUR) reported by Zhang and Xu (2011).
Some halogenated nucleosides were synthesized by BbPyNP under optimized conditions. As shown in Table 5, several 5-halogenated pyrimidine nucleosides were synthesized with the conversions of 5-fluorouridine (83.8%), 5-bromouridine (69%), 5-chlorouridine (74%), FDUR (77%), 5-chloro-2′- deoxyuridine (64%), and 5-bromo-2′-deoxyuridine (60.6%). The conversion of 5-fluorouracil was higher than that of 5-chlorouracil and 5-bromouracil. These differences in conversions may be due to the steric hindrance of halogens, and similar results were reported by Rivero et al. (2012). The conversions of 5-halogenated-2′-deoxynucleosides were higher than that of 5-halogenated nucleosides. This may be due to higher phosphorolysis of deoxynucleosides than that of nucleosides in the reversible reactions. The biosynthesis of 5-halo pyrimidine nucleosides by PyNP from B. subtilis reached a yield of 76% (Serra et al. 2013). Thymidine phosphorylase from E. coli catalyzed the synthesis of FDUR with 73% conversion (Serra et al. 2013). PyNP from Bacillus stearothermophilus JTS859 was able to synthesize FDUR with the yield of 53% (Hori et al. 1990). Immobilized microorganisms Aeromonas salmonicida ATCC 27013 biosynthesis of FDUR reached an 80% conversion (Rivero et al. 2012). The 5-halo pyrimidine nucleosides reached the conversion of 60.6–83.8% by BbPyNP under preliminarily optimized conditions.
Although BbPyNP showed good thermostability, the catalytic activity of BbPyNP should be further improved via molecular modification, such as semi-rational design or directed evolution. Further studies are contemplated to modify the molecular structure of BbPyNP and immobilize the effective mutants.
In conclusion, a PyNP from Brevibacillus borstelensis LK01 was obtained by gene mining. BbPyNP exhibited the advantages of good thermostability and broad range of alkaline pH tolerance. Efficient biosynthesis of several 5-halogenated pyrimidine nucleosides with considerable conversion was achieved by BbPyNP, including 5-fluorouridine, 5-bromouridine, 5-chlorouridine, FDUR, 5-chloro-2′-deoxyuridine, and 5-bromo-2′-deoxyuridine. This research presented an interesting example of mining thermostable PyNP from mesophilic bacteria for biosynthesis of halogenated pyrimidine nucleosides.
References
Balaev VV et al (2016) Substrate specificity of pyrimidine nucleoside phosphorylases of NP-II family probed by X-ray crystallography and molecular modeling. Crystallogr Rep 61:830–841
Grem JL (2000) 5-Fluorouracil: forty-plus and still ticking. A review of its preclinical and clinical development. Invest New Drugs 18:299–313
Hori N, Watanabe M, Yamazaki Y, Mikami Y (1990) Purification and characterization of thermostable pyrimidine nucleoside phosphorylase from Bacillus stearothermophilus JTS 859. Agric Biol Chem 54:763–768
Liu P, Sharon A, Chu CK (2009) Fluorinated nucleosides: synthesis and biological implication. ChemInform 129:743–766
Mikhailopulo IA, Miroshnikov AI (2010) New trends in nucleoside biotechnology. Acta Naturae 2:36
Okuyama K, Hamamoto T, Noguchi T, Midorikawa Y (1996) Molecular cloning and expression of the pyrimidine nucleoside phosphorylase gene from Bacillus stearothermophilus TH 6–2. Biosci Biotechnol Biochem 60:1655–1659
Qian X, Liu B, Wu Q, Lv D, Lin XF (2008) Facile synthesis of novel mutual derivatives of nucleosides and pyrimidines by regioselectively chemo-enzymatic protocol. Bioorg Med Chem 16:5181–5188
Rivero CW, Britos CN, Lozano ME, Sinisterra JV, Trelles JA (2012) Green biosynthesis of floxuridine by immobilized microorganisms. FEMS Microbiol Lett 331:31–36
Ruan Q, Zhou C, Xu X, Wu W (2003) Purification and characterization of a uridine phosphorylase from Enterobacter aerogenes EAM-Z1. Acta Microbiol Sin 43:354–360
Serra I, Bavaro T, Cecchini DA, Daly S, Albertini AM, Terreni M, Ubiali D (2013) A comparison between immobilized pyrimidine nucleoside phosphorylase from Bacillus subtilis and thymidine phosphorylase from Escherichia coli in the synthesis of 5-substituted pyrimidine 2′-deoxyribonucleosides. J Mol Catal B Enzym 95:16–22
Szeker K, Zhou X, Schwab T, Casanueva A, Cowan D, Mikhailopulo IA, Neubauer P (2012) Comparative investigations on thermostable pyrimidine nucleoside phosphorylases from Geobacillus thermoglucosidasius and Thermus thermophilus. J Mol Catal B-enzym 84:27–34
Taran SA, Verevkina KN, Feofanov SA, Miroshnikov AI (2009) Enzymatic transglycosylation of natural and modified nucleosides by immobilized thermostable nucleoside phosphorylases from Geobacillus stearothermophilus. Bioorg Khim 35:822–829
Van Rompay AR, Johansson M, Karlsson A (2003) Substrate specificity and phosphorylation of antiviral and anticancer nucleoside analogues by human deoxyribonucleoside kinases and ribonucleoside kinases. Pharmacol Ther 100:119
Zhang X, Xu YZ (2011) NMR and UV studies of 4-thio-2′-deoxyuridine and its derivatives. Mol 16:5655–5664
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81673321, 21376119, 21506099), and Natural Science Foundation of the Jiangsu Higher Education Institution of China (15KJB530008).
Supporting information
Supplementary Table 1—The primers used in the experiments.
Supplementary Fig. 1—Mass spectrometry analysis of 5-fluoro-2′-deoxyuridine.
Supplementary Fig. 2—1H-NMR spectroscopic data of 5-fluoro-2′-deoxyuridine.
Supplementary Fig. 3—13C-NMR spectroscopic data of 5-fluoro-2′-deoxyuridine.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, K., Zhou, Y., Zhang, J. et al. A thermostable pyrimidine nucleoside phosphorylase from Brevibacillus borstelensis LK01 for synthesizing halogenated nucleosides. Biotechnol Lett 39, 1903–1910 (2017). https://doi.org/10.1007/s10529-017-2423-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2423-1