Abstract
Objective
To remove dibenzothiophene (DBT) and 4,6-dimethyl-dibenzothiophene (4,6-DMDBT) adsorbed on alumina, silica and sepiolite through biodesulfurization (BDS) using Rhodococcus Rhodochrous spp., that selectively reduce sulfur molecules without generating of gaseous pollutants.
Results
The adsorption of DBT and 4,6-DMDBT was affected by the properties of the supports, including particle size and the presence of surface acidic groups. The highest adsorption of both sulfur-containing organic molecules used particle sizes of 0.43–0.063 mm. The highest percentage removal was with sepiolite (80 % for DBT and 56 % for 4,6-DMDBT) and silica (71 % for DBT and 37 % for 4,6-DMDBT). This is attributed to the close interaction between these supports and the bacteria.
Conclusions
Biodesulfurization is effective for removing the sulfur-containing organic molecules adsorbed on inorganic materials and avoids the generation of gaseous pollutants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The elimination of sulfur-containing organic molecules present in fuel is carried out through a hydrodesulfurization (HDS) reaction (Ojeda et al. 2005). This process can desulfurize compounds like thiophene, benzothiophene, and dibenzothiophene (DBT), but it is unsuccessful in treating dibenzothiophene derivatives that have alkyl groups near the sulfur atom, especially 4,6-dimethyldibenzothiophene (4,6-DMDBT) (Baeza et al. 2015). In this context, adsorptive desulfurization is a promising alternative approach to remove sulfur-containing organic molecules using inorganic materials as adsorbents (Kilbane 2006). Despite numerous studies on the adsorptive desulfurization process, it still possesses unresolved difficulties, the foremost of which is the regeneration of the adsorbent material. This is because adsorbent regeneration releases SO2 into the environment (Li et al. 2009). In this context the biodesulfurization (BDS) (Li et al. 2006), which is a biocatalytic process performed by microorganisms that remove selectively sulfur from hydrocarbon fractions without the generation of gaseous pollutants, is an alternative that could be applied for the elimination of the adsorbed sulfur molecules. In this regard, this work has evaluated the environmentally-friendly removal of sulfur-containing organic molecules adsorbed on inorganic materials through the use of BDS. Also, the influence of physical parameters of the inorganic materials on the processes of adsorption and removal of sulfur-containing organic molecules was studied.
Materials and methods
Bacterial strain
Rhodococcus rhodochrous (ATCC 53968) was grown in sulfur-free Medium A (Maghsoudi et al. 2001) containing sodium succinate (30 mM) and citrate (0.1 % w/v) as energy and carbon sources, respectively. Dibenzothiophene (DBT) (62 mM) (Merck) was used as the only sulfur source.
Sulfur-containing organic molecules and support materials
DBT (100 mg l−1) and 4,6-dimethyldibenzothiophene (4,6-DMDBT) (100 mg l−1) were used as sulfur-containing organic molecules. Silica (Si) D11-10 BASF (specific area of 80 m2 g−1), alumina (Al) T-126 Girdler (specific area of 200 m2 g−1), and sepiolite (Sep) 120 NF Tolsa (specific area of 300 m2 g−1) were used as inorganic supports (Dinamarca et al. 2014).
Selection of support particle size and determination of adsorption of sulfur-containing organic molecules
To study the effect of the particle size of the support on biodesulfurization (BDS), DBT and 4,6-DMDBT were mixed with Si, Al or Sep with two different particle size distributions of 0.063–0.425 and 1.18–2 mm in 25 ml flasks at 25 °C and shaken at 200 rpm for 24 h. To study the adsorption of sulfur-containing organic molecules, each support (1 g) was placed in a 25 ml flask containing 10 ml DBT (100 mg l−1) or 4,6-DMDBT (100 mg l−1) and mixed for 80 h. Samples of the solution were taken for analysis during the adsorption process. The amounts of adsorbed sulfur-containing organic molecules were analyzed by GC (Baeza et al. 2015).
Adsorption kinetics
The adsorption kinetics of DBT and 4,6-DMDBT over the three different support materials were studied considering non-dissociating molecular adsorption of these molecules on the support materials. The quantity adsorbed at any given time (qt) and equilibrium adsorption capacity (qe), could be predicted by either a pseudo-first-order or pseudo-second-order kinetic model (Srivastav and Srivastava 2009). The experimental data were fitted with the two models to determine the model kinetic parameters by non-linear regression analysis using Origin 8.0 statistical software.
Removal of adsorbed sulfur-containing organic molecules
To remove the adsorbed sulfur-containing organic molecules, free cells, collected by centrifugation at 4000×g for 30 min at 4 °C (from 109 to 25 × 109 cells), were placed in a 25 ml flask containing sulfur-free Medium A (10 ml) with 1 g support containing adsorbed DBT or 4,6-DMDBT. The reaction was carried out at 30 °C at 200 rpm for 48 h. The cultures were centrifuged and the residual sulfur-containing organic molecules adsorbed on the supports were extracted with ethyl acetate. The contents of DBT and 4,6-DMDBT were analyzed by GC (Baeza et al. 2015). The systems are designated as sulfur-containing organic molecule/solid systems, where the sulfur-containing organic molecules were DBT or 4,6-DMDBT, and the solid was the inorganic support. The activity of each experiment is expressed as the percentage removal of sulfur-containing organic molecules.
Results and discussion
Selection of support particle size
The results (Table 1) reveal that higher adsorption of both sulfur-containing organic molecules occurred when a solid support with particle sizes from 0.43 to 0.063 mm was used as adsorbent; i.e., the adsorption of dibenzothiophene (DBT) and 4,6-dimethyl-dibenzothiophene (4,6-DMDBT) increased as the particle size of the adsorbents decreased. A larger total surface area per volume and a shorter diffusion path for the adsorbate when using smaller particles can explain this behavior (Tsai et al. 2003; Lu et al. 2016). On the other hand, the differences observed in the adsorption values of DBT and 4,6-DMDBT on the different supports at 1 and 24 h, showed in the Table 1, are caused by the different interactions between these sulfur-containing organic molecules with the adsorbents (Kim et al. 2006).
Adsorption of sulfur-containing organic molecules
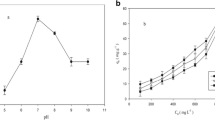
The adsorption capacity of DBT on the different supports is shown in Fig. 1a. At the beginning of the adsorption process, all supports displayed a linear increase of DBT adsorption until a maximum value reached after approximately 10 h. At longer times the adsorption values then remained constant until 80 h. The maximum adsorption of DBT on silica (Si) was 35.5 mg l−1, while those on alumina (Al) and sepiolite (Sep) were 30 and 23 mg l−1, respectively. In the case of adsorption of 4,6-DMDBT, as illustrated in Fig. 1b, only Si exhibited similar behavior to DBT adsorption, while maximum adsorption on Sep and Al was reached after longer periods. Comparison of the adsorption of both sulfur-containing organic molecules revealed higher adsorption of DBT on Si and Al, while no marked difference was observed in the case of Sep. The variation of the adsorption of DBT and 4,6-DMDBT on the supports can be explained by the different specific areas and density and strength of acid sites of these materials (Kim et al. 2006). However, the higher adsorption of both sulfur-containing organic molecules on the Si support showed that the strength of acid sites is the main factor that affects the adsorption of recalcitrant sulfur-containing molecules.
a Adsorption capacity of dibenzothiophene (DBT) on Alumina (Al), silica (Si) and sepiolite (Sep). (Filled cirle DBT/Sep, triangle DBT/Al, square DBT/Si). b Adsorption capacity of 4,6-dimethyldibenzothiophene (4,6-DMDBT) on Alumina (Al), silica (Si) and sepiolite (Sep). (Filled cirle 4,6-DMDBT/Sep, triangle 4,6-DMDBT/Al, square 4,6-DMDBT/Si)
Table 2 summarizes the kinetic rate constants obtained using the first- and second-order models (kf and ks, respectively). Considering the three support materials studied, the highest values for the coefficient of determination (r2) and lowest values of the residual sum of squares (RSS) suggest that the adsorption of DBT and 4,6-DMDBT follow second-order kinetics. The qe values of the samples (Table 2) confirm that the strongest interaction of DBT is with Sep while for the case of 4,6-DMDBT is with Si.
Removal of adsorbed sulfur-containing organic molecules by Rhodococcus rhodochrous
Table 3 presents the percentage removal of the sulfur-containing organic materials adsorbed on supports by BDS; the removal of DBT and 4,6-DMDBT clearly depends on the type of support. Higher degradation of both sulfur-containing organic materials was observed when Sep and Si were used as the. The differences observed for the removal of the sulfur-containing compounds by the same support can be explained by the different interactions between the cells and supports (Dinamarca et al. 2010). Both physical and biological factors can influence cell–support interactions, such as the size of the bacterial cells, ionic strength (Yee et al. 2000) and the support surface structure (Jeyachandran et al. 2006). We previously studied the BDS activity of gas oil of biocatalytic immobilized systems using Pseudomonas stutzeri adsorbed on inorganic materials (Dinamarca et al. 2010). Our results showed higher activity in systems that had stronger interactions between the bacterial cells and support. The greater removal of DBT compared with that of 4,6-DMDBT could be explained by the difficulty microorganisms have desulfurizing long-chain alkylated DBTs (Bhatia and Sharma 2010).
Conclusions
Biodesulfurization (BDS) is effective and can be used to remove sulfur-containing organic molecules adsorbed on inorganic materials and avoid the generation of gaseous pollutants. The efficient removal of sulfur-containing organic molecules depends on the interaction between the support and adsorbent and the adsorption capacity of the bacteria used.
References
Baeza P, Bassi R, Villarroel M, Ojeda J, Araya P, Aguila G (2015) Adsorption of 4,6-dimethyldibenzothiophene over Cu/ZrO2. J Chil. Chem Soc 60:2817–2821
Bhatia S, Sharma DK (2010) Biodesulfurization of dibenzothiophene, its alkylated derivatives and crude oil by a newly isolated strain Pantoea agglomerans D23W3. Biochem Eng J 50:104–109
Dinamarca MA, Ibacache-Quiroga C, Baeza P, Galvez S, Villarroel M, Ojeda J (2010) Biodesulfurization of gas oil using inorganic supports biomodified with metabolically active cells immobilized by adsorption. Biores Technol 101:2375–2378
Dinamarca MA, Rojas A, Baeza P, Espinoza G, Ibacache-Quiroga C, Ojeda J (2014) Optimizing the biodesulfurization of gas oil by adding surfactants to immobilized cell systems. Fuel 116:237–241
Jeyachandran YL, Narayandassa SK, Mangalaraj D, Bao CY, Li W, Liao YM, Zhang C, Xiao LY, Chen WC (2006) A study on bacterial attachment on titanium and hydroxyapatite based films. Surf Coat Technol 201:3462–3474
Kilbane JJ II (2006) Microbial biocatalyst developments to upgrade fossil fuels. Curr Opin Biotechnol 17:305–314
Kim JH, Ma X, Zhou A, Song C (2006) Ultra-deep desulfurization and denitrogenation of diesel fuel by selective adsorption over three different adsorbents: a study on adsorptive selectivity and mechanism. Catal Today 111:74–83
Li W, Wang MD, Chen H, Shi Y (2006) Biodesulfurization of dibenzothiophene by growing cells of Gordonia sp. in batch cultures. Biotechnol Lett 28:1175–1179
Li W, Tang W, Liu Q, Xing J, Li Q, Wang D, Yang M, Li X, Liu H (2009) Deep desulfurization of diesel by integrating adsorption and microbial method. Biochem Eng J 44:297–301
Lu MC, Charisse LC, Wan MW, De Leon R, Arco S, Futalan C (2016) Adsorption of dibenzothiophene sulfone from fuel using chitosan-coated bentonite (CCB) as biosorbent. Desalin Water Treat 57:5108–5118
Maghsoudi S, Vossoughi M, Kheirolomoom A, Tanaka E, Katoh S (2001) Biodesulfurization of hydrocarbons and diesel fuels by Rhodococcus sp. strain P32C1. Biochem Eng J 8:151–156
Ojeda J, Escalona N, Fierro JLG, López-Agudo A, Gil-Llambías FG (2005) Effect of the preparation of Re/γ-Al2SO3 catalysts on the HDS and HDN of gasoil. Appl Catal A 281:25–30
Srivastav A, Srivastava VC (2009) Adsorptive desulfurization by activated alumina. J Hazard Mater 170:1133–1140
Tsai WT, Lai CW, Hsien KJ (2003) Effect of particle size of activated clay on the adsorption of paraquat from aqueous solution. J Colloid Interface Sci 263:29–34
Yee N, Fein JB, Daughney JC (2000) Experimental study of the pH, ionic strength, and reversibility behavior of bacteria–mineral adsorption. Geochim Cosmochim Ac 4:609
Acknowledgments
The authors are grateful to the Chilean government for financial support from CONICYT through FONDECYT Grant 1150544.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carvajal, P., Dinamarca, M.A., Baeza, P. et al. Removal of sulfur-containing organic molecules adsorbed on inorganic supports by Rhodococcus Rhodochrous spp.. Biotechnol Lett 39, 241–245 (2017). https://doi.org/10.1007/s10529-016-2240-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2240-y