Abstract
Objectives
To demonstrate biotransformation of toxic Cr(VI) ions into Cr2O3 nanoparticles by the yeast Schwanniomyces occidentalis.
Results
Reaction mixtures containing S. occidentalis NCIM 3459 and Cr(VI) ions that were initially yellow turned green after 48 h incubation. The coloration was due to the synthesis of chromium (III) oxide nanoparticles (Cr2O3NPs). UV–Visible spectra of the reaction mixtures showed peaks at 445 and 600 nm indicating 4A2g → 4T1g and 4A2g → 4T2g transitions in Cr2O3, respectively. FTIR profiles suggested the involvement of carboxyl and amide groups in nanoparticle synthesis and stabilization. The Cr2O3NPs ranged between 10 and 60 nm. Their crystalline nature was evident from the selective area electron diffraction and X-ray diffraction patterns. Energy dispersive spectra confirmed the chemical composition of the nanoparticles. These biogenic nanoparticles could find applications in different fields.
Conclusions
S. occidentalis mediated biotransformation of toxic Cr(VI) ions into crystalline extracellular Cr2O3NPs under benign conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A variety of metal ions enter aquatic environments via natural processes and human activities. Chromium is one such toxic metal that is regarded as a priority pollutant. In nature, chromium exists in different oxidation states and the trivalent [Cr(III)] and hexavalent [Cr(VI)] forms are the most prevalent. Hexavalent chromium [Cr(VI)] negatively affects human health and there is a need to treat this effectively. In addition to the conventional physical and chemical treatment methods that are available, biological protocols for addressing this problem have also been described (Wang et al. 2008; Yong et al. 2015). Development of mild processes for detoxifying Cr(VI) ions with simultaneous production of value-added products such as Cr2O3 nanoparticles could be a promising way of tackling this issue.
Cr2O3 nanoparticles (NP) are extensively used as catalysts, coating material and pigments. They are usually synthesized by physical and chemical methods. There are recent reviews highlighting the significance of microorganisms in synthesizing metal nanoparticles (Park et al. 2015; Yadav et al. 2015). However, there are very few studies on the production of Cr2O3NPs by microbial systems (Dong et al. 2013; Annamalai et al. 2014; Wang et al. 2013). These reports describe the production of “cell associated” Cr2O3NPs by bacterial isolates. To the best of our knowledge there are no reports on extracellular synthesis of Cr2O3NPs by yeasts. In the current investigation, we describe (i) biotransformation of toxic hexavalent potassium chromate (K2CrO4) to extracellular Cr2O3 nanoparticles by the yeast Schwanniomyces occidentalis (ii) detail the characteristics of these biogenic nanoparticles by a variety of analytical techniques and (iii) propose their eventual use in different fields.
Materials and methods
Biotransformation of hexavalent chromium ions
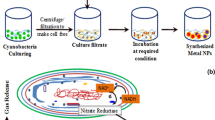
Schwanniomyces occidentalis NCIM 3459 was inoculated in 20 ml Burkholder’s mineral medium (Ksheminska et al. 2006) containing 10 mM K2CrO4 and incubated under static conditions at 30 °C for 10 days. A color change (yellow to deep green) indicated the biotransformation of Cr(VI) ions and the probable formation of Cr2O3 nanoparticles. The effect of temperature (10–50 °C) and salt concentration (2.5–12.5 mM) on nanoparticle synthesis was monitored. After incubation, the reaction mixtures were centrifuged at 6000×g for 10 min and Cr2O3NPs in the supernatant were characterized.
For all experiments, the source of Cr(VI) ions was K2Cr2O4 in distilled water. The total Cr content in the cell free extract (CFE) and cells was determined by using model SpectrAA220 Atomic Absorption Spectrometer (AAS). The content of residual Cr(VI) ions in the CFE was determined spectrophotometrically by the diphenyl carbazide method. Differences between total chromium and Cr(VI) concentrations were used to determine the Cr(III) contents.
Fourier transform infrared (FTIR) analysis
The functional groups involved in nanoparticle synthesis were detected by FTIR analysis. The IR spectra of KBr-pelleted nanoparticle samples were recorded in the range of 500–4000 cm−1 at a resolution of 4 cm−1 on a Jasco FTIR-6100 model.
Characterization of the biogenic nanoparticles
Properties of the extracellular nanoparticles were determined by different analytical tools. UV–Visible spectra were recorded at a resolution of 1 nm. transmission electron microscope (TEM) observations were made on FEI model, Tecnai G2 20 TEM equipped with selected area electron diffraction (SAED) analyzer. Samples were prepared by coating nanoparticles on carbon-coated copper grids (200 µm × 200 µm mesh size) and drying them prior to observation. X-ray diffraction (XRD) patterns were obtained by using D8 Advanced Brucker X-ray diffractometer with Ni-filtered Cu–Kα X-rays of wavelength (λ) 1.540 A°. Thin films of the nanoparticles were coated on glass slides and X-ray diffraction patterns were recorded in the 2θ range of 20–80° with the step size of 0.05 and step time of 1 s in the transmission mode. The energy dispersive spectrometer (EDS) attachment present with the model FEI Nova NanoSEM 450 field emission scanning electron microscope (FESEM) was used to obtain EDS profiles.
All experiments were carried out in triplicate with two biological replicates and representative spectra, data and images are presented here.
Results and discussion
Biotransformation of hexavalent chromium ions by Schwanniomyces occidentalis
During our preliminary investigations on biotransformation of Cr(VI) ions, we identified the ability of this strain of S. occidentalis in biotransforming potassium chromate. S. occidentalis has been used in beer-making and production of single cell protein (Horn et al. 1992; Anupama and Ravindra 2000). Considerable biomass of the yeast is thus generated and this can be used for the application described in the current investigation. To the best of our knowledge, there are no reports on the use of the biomass of this yeast for preparation of chromium oxide nanoparticles in an extracellular manner and hence this study was taken up.
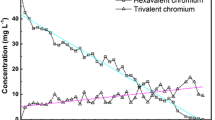
When S. occidentalis was incubated with 10 mM K2CrO4 under static conditions, the reaction mixtures turned from bright yellow to deep green (Fig. 1a, tubes C and T, respectively) indicating the reduction of the hexavalent form into the trivalent form. This color change typically depicts the presence of Cr2O3NPs (Willis et al. 2007). When these reaction mixtures were centrifuged, color was associated with the CFE and not with cell pellets indicating the extracellular nature of the nanoparticles. Cr(VI) ions in the reaction mixtures decreased in a time-dependent manner and were below detectable limit after 30 h of incubation (Fig. 1a). AAS analysis revealed that 98 % of the initially added chromium was present in the cell free extract (CFE). Since Cr(VI) ions were not detected in the CFE, it was clear that the cells mediated the reduction of the hexavalent form into the trivalent form as Cr2O3NPs. There is a report on a Bacillus species bringing about reduction of Cr(VI) ions to the Cr(III) form into without being accumulated in the cell (Elangovan et al. 2006). In a similar manner, S. occidentalis seems to be mediating the chromium reduction reactions in an extracellular manner.
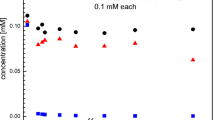
Biotransformation of Cr(VI) ions by Schwanniomyces occidentalis. a Residual Cr(VI) ions in reaction mixtures over a period of time after biotransformation by S. occidentalis [Inset tube C (10 mM K2CrO4 without yeasts) and T (10 mM K2CrO4 with yeasts)]. b UV–Visible spectra and visual observations (insets) of reaction mixtures depicting effect of K2CrO4 concentration on nanoparticle synthesis [lines and tubes 1 2.5, 2 5.0, 3 7.5, 4 10 and 5 12.5 mM]. Intended for color reproduction on the Web (free of charge) and in print or to be reproduced in color on the Web (free of charge) and in black‐and‐white in print
The intensity of the color increased with increasing content of K2CrO4 (Fig. 1b, inset tubes 1–5). Control experiments (containing medium and K2CrO4) did not show any color change indicating that the yeast cells were mediating the reductive reactions (Fig. 1a, tube C). UV–Visible spectra of the reaction mixtures were also obtained (Fig. 1b, lines 1–5). Peaks were observed in the vicinity of 445 and 600 nm (Fig. 1b, black arrows) corresponding to the 4A2g → 4T1g and 4A2g → 4T2g transitions in Cr2O3, respectively (Li et al. 2006). The peak at around 445 nm is associated with the inter-band transition of core electrons of chromium to chromium oxide (Ramesh et al. 2012). These results validate the earlier observations on the conversion of Cr(VI) ions into Cr2O3 that were based on AAS quantification of total chromium and spectrophotometric detection of Cr(VI) ions. Waste-waters often contain high contents of Cr(VI) ions. Incubation of such wastewaters with this yeast could thus (i) mediate effective detoxification and (ii) generate value-added products in the form of Cr2O3NPs.
The effect of temperature on nanoparticle synthesis was also determined. When reaction mixtures were incubated at 10, 20, or 30 °C, color transition was observed after 10 days. Incubation at 40 and 50 °C decreased the reaction times to 6 and 2 days, respectively. In the current study, synthesis of nanoparticles was achieved in shorter time durations at higher temperatures.
It is hypothesized that incubation at 50 °C favored the release of biomolecules involved in the reduction and stabilization of nanoparticles. S. occidentalis is known to grow in the presence of Cr(VI) ions and produce higher contents of riboflavin (Fedorovych et al. 2001). The yeast thus has the necessary set up to tolerate and detoxify Cr(VI) ions. As discussed in the following section, TEM images showed the presence of an organic layer [that could be derived from the extracellular polymeric substance (EPS)] capping the nanoparticles. Capping agents naturally restrict nanoparticles from growing further. Higher temperatures probably favored enhanced synthesis of the EPS and allowed the capping to occur more rapidly.
FTIR analysis
To identify the functional groups involved in nanoparticle synthesis, FTIR analysis was carried out. FTIR spectra of control (untreated with K2CrO4) and test reactions (treated with K2CrO4) are depicted in Fig. 2 (lines 1 and 2, respectively). Arrows and wave numbers in the figure highlight important peaks involved in nanoparticle synthesis. Stretching of the peaks at 3400, 1590–1600, 1395–1440 and 1078 cm−1 could be assigned to the –OH bond, primary amide bond, carboxylic acid residues and C–O–C and C–O–P ring vibrations of polysaccharides, respectively (Omoike and Chorover 2004). Peaks at 885–895 corresponded to the –C–H stretching and the peak at around 800 cm−1 indicated the role of amine groups. FTIR spectra thus suggested the role of carbohydrate and protein moieties in the synthesis and stabilization of nanoparticles.
Characterization of extracellular Cr2O3NP nanoparticles
TEM images of the nanoparticles at different magnifications are depicted in Fig. 3a, b. The Cr2O3NP varied from 10 to 60 nm. The nanoparticles (Fig. 3a, b, single black arrow) were embedded in an organic matrix probably derived from the EPS (Fig. 3a, b, double black arrows). The role of EPS in formation of silver nanoparticles has also been described with respect to Exiguobacterium mexicanum (Padman et al. 2014). SAED patterns of the Cr2O3NP (Fig. 3c) showed the presence of diffraction rings and spots indicating the polycrystalline nature of the nanoparticles.
To further characterize the crystal structure of the nanoparticles, XRD studies were taken up. Sharp peaks were obtained at 2θ values of 24.65°, 41.46° and, 50.28° (Fig. 4a). These peaks could be indexed to the (0 1 2), (1 1 3) and (0 2 4) crystalline planes of Cr2O3NPs, respectively (Tavares et al. 2014). A representative spot-EDS profile of Cr2O3NPs displayed in addition to other peaks, distinct signals for chromium and oxygen (Fig. 4b, black arrows).
Characterization of Cr2O3NPs synthesized by Schwanniomyces occidentalis. a Representative X-ray diffraction profile [sharp peaks at 2θ values of 24.65°, 41.46° and, 50.28º were indexed to the (0 1 2), (1 1 3) and (0 2 4) crystalline planes of Cr2O3NPs, respectively]. b Representative energy dispersive spectrum showing in addition to other peaks, distinct signals for chromium and oxygen [indicated by black and gray arrows, respectively]
S. occidentalis thus effectively biotransformed toxic Cr(VI) ions into value-added products in the form of extracellular Cr2O3NPs. As stated earlier, there are very few reports on the synthesis of Cr2O3NPs by microbial systems. There are reports on bacteria such as Shewanella oneidensis, Bacillus cereus and Bacillus subtilis converting Cr(VI) ions to Cr2O3NPs that are cell associated (Wang et al. 2013; Dong et al. 2013; Annamalai et al. 2014). Since such nanoparticles are closely linked to cells, their applications are limited. To the best of our knowledge, there are no reports on yeasts mediating such reactions. The yeast culture used in the current study offered a major advantage by synthesizing nanoparticles extracellularly. FTIR data suggested the role of carbohydrate and protein moieties in mediating the synthesis and capping of nanoparticles (Fig. 2). Moreover, TEM images also revealed the presence of a polymeric layer around the nanoparticles. The yeast thus produced EPS that mediated the reductive reactions and stabilized the nanoparticles. The significance of EPS in chromium reduction reactions has also been highlighted in a recent review (Thatoi et al. 2014).
The advantages associated with the current biogenic protocol are two-fold. The yeast cells could transform Cr(VI) ions present in high concentrations (as in wastewaters) under static and benign conditions thus making the technique cost-effective. Moreover, the end products generated as a result of the biotransformation process were value-added products. It must be noted that nanostructured Cr2O3 materials offer high surface areas and are thus used in catalysis, as green pigments and as coating material.
Conclusion
A simple eco-friendly method of biotransforming toxic hexavalent chromium ions into Cr2O3NPs by using the yeast Schwanniomyces occidentalis is described. The yeast formed extracellular crystalline Cr2O3NPs under mild conditions without the use of toxic or expensive chemicals or high-end equipment. This process could be made cost-effective and could be scaled-up by employing yeast biomass obtained from industrial processes and by using Cr(VI) ions-containing wastewaters. Work on characterization of the EPS is on-going.
References
Annamalai K, Nair AM, Chinnaraju S, Kuppusamy S (2014) Removal of chromium from contaminated effluent and simultaneously green nanoparticle synthesis using Bacillus subtilis. Malaya J Biosci 1:13–18
Anupama, Ravindra P (2000) Value-added food: single cell protein. Biotechnol Adv 8:459–479
Dong G, Wang Y, Gong L, Wang M, Wang H, He N, Zheng Y, Li Q (2013) Formation of soluble Cr(III) end-products and nanoparticles during Cr(VI) reduction by Bacillus cereus strain XMCr–6. Biochem Eng J 70:166–172
Elangovan R, Abhipsa S, Rohit B, Ligy P, Chandraraj K (2006) Reduction of Cr(VI) by a Bacillus sp. Biotechnol Lett 28:247–252
Fedorovych D, Kszeminska H, Babjak L, Kaszycki P, Kołoczek H (2001) Hexavalent chromium stimulation of riboflavin synthesis in flavinogenic yeast. Biometals 14:23–31
Horn CH, du Preez JC, Kilian SG (1992) Fermentation of grain sorghum starch by co-cultivation of Schwanniomyces occidentalis and Saccharomyces cerevisiae. Bioresour Technol 42:27–31
Ksheminska HP, Honchar TM, Gayda GZ (2006) Extra-cellular chromate-reducing activity of the yeast cultures. Cent Eur J Biol 1:137–139
Li L, Yan ZF, Lu GQ, Zhu ZH (2006) Synthesis and structure characterization of chromium oxide prepared by solid thermal decomposition reaction. J Phys Chem B 110:178–183
Padman AJ, Henderson J, Hodgson S, Rahman PKSM (2014) Biomediated synthesis of silver nanoparticles using Exiguobacterium mexicanum. Biotechnol Lett 36:2079–2084
Park TJ, Lee KG, Lee SY (2015) Advances in microbial biosynthesis of metal nanoparticles. Appl Microbiol Biotechnol. doi:10.1007/s00253-015-6904-7
Ramesh C, Mohankumar K, Latha N, Ragunathan V (2012) Green synthesis of Cr2O3 nanoparticles using Tridax procumbens leaf extract and its antibacterial activity on Escherichia coli. Curr Nanosci 8:603–607
Sallau AB, Inuwa HM, Ibrahim S, Nok AJ (2014) Isolation and properties of chromate reductase from Aspergillus niger. Int J Mod Cell Mol Biol 3:10–21
Tavares KP, Caloto-Oliveira Á, Vicentini DS, Melegari SP, Matias WG, Barbosa S, Kummrow F (2014) Acute toxicity of copper and chromium oxide nanoparticles to Daphnia similis. Ecotoxicol Environ Contam 9:943–950
Thatoi H, Das S, Mishra J, Rath BP, Das N (2014) Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: a review. J Environ Manag 146:383–399
Wang G, Huang L, Zhang Y (2008) Cathodic reduction of hexavalent chromium [Cr(VI)] coupled with electricity generation in microbial fuel cells. Biotechnol Lett 30:1959–1966
Wang Y, Sevinc PC, Belchik SM, Fredrickson J, Shi L, Lu HP (2013) Single-cell imaging and spectroscopic analyses of Cr(VI) reduction on the surface of bacterial cells. Langmuir 29:950–956
Yadav A, Kon K, Kratosova G, Duran N, Ingle AP, Rai M (2015) Fungi as an efficient mycosystem for the synthesis of metal nanoparticles: progress and key aspects of research. Biotechnol Lett 37:2099–2120
Yong P, Liu W, Zhang Z, Beauregard D, Johns ML, Macaskie LE (2015) One step bioconversion of waste precious metals into Serratia biofilm-immobilized catalyst for Cr(VI) reduction. Biotechnol Lett 37:2181–2191
Acknowledgments
PM wishes to thank the Council of Scientific and Industrial Research, India for Senior Research Fellowship. All authors thank University Grants Commission, India for support under University with Potential for Excellence Phase II.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohite, P.T., Kumar, A.R. & Zinjarde, S.S. Biotransformation of hexavalent chromium into extracellular chromium(III) oxide nanoparticles using Schwanniomyces occidentalis . Biotechnol Lett 38, 441–446 (2016). https://doi.org/10.1007/s10529-015-2009-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-015-2009-8