Abstract
Hemostasis in orthopedic osteotomy or bone cutting requires different methods and materials. The bleeding of bone marrow can be mostly stopped by bone wax. However, the wax cannot be absorbed, which leads to artificial prosthesis loosening, foreign matter reaction, and infection. Here, a bioactive glass/chitosan/carboxymethyl cellulose (BG/CS/CMC) composite scaffold was designed to replace traditional wax. WST-1 assay indicated the BG/CS/CMC composite resulted in excellent biocompatibility with no cytotoxicity. In vivo osteogenesis assessment revealed that the BG/CS/CMC composite played a dominant role in bone regeneration and hemostasis. The BG/CS/CMC composite had the same hemostasis effect as bone wax; in addition its biodegradation also led to the functional reconstruction of bone defects. Thus, BG/CS/CMC scaffolds can serve as a potential material for bone repair and hemostasis in critical-sized bone defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone wax has been used for more than a century as a classic hemostasis material of bone osteotomy or bone cutting. Due to its immediacy and effectiveness of hemostasis, ease of use and low cost, it is still widely used in surgical procedures. Although it is an effective hemostatic device, which creates a physical barrier to stop bleeding, bone wax remains at the implantation site indefinitely and influences subsequent bone growth (Vestergaard et al. 2010). Moreover, it has a potential risk of infection in clinical applications (Gibbs et al. 2004). Thus, it is desirable to devise an alternative, topical hemostatic agent with good histocompatibility, osteoconduction, suitable degradation rate and porosity.
Chitosan is substitute material for bone defects because of its high biocompatibility, osteoconductivity, antimicrobial, biodegradability and adsorption properties (Zhang et al. 2012). Bioactive glass can bond spontaneously to living bones without forming fibrous tissue at the interface (Shi et al. 2007). Although the formation of pores in bioactive glass may adversely affect its mechanical properties, the advantages provided by the pores are crucial in repairing bone defects (Lew et al. 2012). Carboxymethyl cellulose (CMC), derived through chemical modification of cellulose, has been studied as an in vivo carrier of demineralized bone matrix with encouraging results (Kim et al. 2005). Despite favorable clinical outcomes, there is limited histological data characterizing the effects of CMC on bone regeneration (Walsh et al. 2003). Oppositely charged chitosan-CMC polysaccharides assembled through electrostatic interaction to form a porous framework (Jiang et al. 2013).
Similar studies to find an ideal alternative for bone wax have been reported. Zhang et al. (2012) suggested that an injectable nanohydroxyapatite/chitosan scaffold was a potential candidate material for regeneration of bone loss. Vestergaard et al. (2010) indicated that the use of Ostene instead of bone wax could contribute to a reduction in the incidence of sternal dehiscence and chronic inflammation. Cho et al. (2012) suggested the application of a thrombin-soaked, absorbable gelatin compressed sponge at the end of multilevel posterior cervical spinal surgery significantly decreased post-operative drain output.
In this study, a bioactive biogas/chitosan/CMC (BG/CS/CMC) composite hydrogel was prepared through gradual electrostatic assembly. Cellular compatibility of the composite was assessed prior to in vivo testing. To investigate the capability of in vivo osteogenesis, scaffolds were implanted into bone defects of New Zealand white rabbits. Osteogenesis assessment was carried out by X-ray photography and histological analysis. In vivo osteogenesis assessment revealed that the BG/CS/CMC composite played a dominant role in bone regeneration and hemostasis with the good biodegradability and had the same hemostasis effect as bone wax. Our findings suggest that BG/CS/CMC scaffolds can serve as a potential material for bone repairing and hemostasis in critical sized bone defects.
Materials and methods
Raw materials
Bioactive glass powder (Biomaterial Co. Ltd, KunShan, China) of 58S (60 mol % SiO2, 36 mol % CaO, 4 mol % P2O5) and 80 mesh chitosan powder (Biotemed Biomaterial Co. Ltd, Qingdao, China) with a molecular weight of ~25 kDa and a N-acetylation degree of 85 % were used. Sodium carboxymethyl cellulose (Sinopharm, China) with a viscosity of 800–1,200 (25 °C) was also used. All other reagents used here were of analytical grade.
Bone wax (Ethicon, USA) is a sterile non-absorbable wax comprising 75 % (w/v) white beeswax, 15 % (w/v) paraffin wax and 10 % (w/v) isopropyl palmitate.
Preparation of BG/CS/CMC composites
BG/CS/CMC composites were synthesized as follows: 5 % (w/v) chitosan was obtained by dissolving 0.2 g chitosan in 4 ml acetic acid (1 % w/v), and the solution was sterilized by filtering through a 0.22 µm filter. Bioactive glass, 0.2 g (sterilized with 250 kGy of γ-radiation from a 60Co source) was added into the chitosan solution. Then 0.75 g CMC (sterilized by irradiation) was added. The bottle with the BG/CS/CMC composites was sealed for 12 h at room temperature. Finally, the mixture was kneaded for 30 min with sterile gloves and the sample was stored at room temperature (20 °C). The obtained waxy composites of the composites were cut into small bars (1 cm × 2 cm × 0.5 cm), and sterilized by UV irradiation for 30 min.
Characterization of BG/CS/CMC composites
The microstructures of BG/CS/CMC composites were observed with a scanning electron microscope. Samples were prepared by lyophilization of the above composites. Based on the quantitative analysis of at least 40 pores, pore size was calculated by ImageJ software.
Cell culture
Synovium-derived mesenchymal cells (SMSC) were isolated and expanded according to De Bari et al. (2001). SMSC were seeded in culture flasks and allowed to proliferate in the complete medium [low-glucose Dulbecco’s modified Eagle’s medium (DMEM) containing 10 % (v/v) fetal bovine serum (FBS) and antibiotics (100 IU/ml penicillin, 100 IU/ml streptomycin) at 37 °C in a humidified atmosphere of 5 % (v/v) CO2]. The complete medium was replaced every 3–4 days. When the attached cells reached 90 % confluence after 9–12 days of primary culture, they were washed twice with sterilized PBS solution, collected by treatment with trypsin/EDTA (0.25 % trypsin, 1 mM EDTA) and seeded in new culture flasks at a dilution rate of 1:4 for the first subculture.
Cell viability
Cell viability was determined using a water-soluble tetrazolium-1 (WST-1) assay kit (Hoffmann-La Roche, New Jersey), which quantifies mitochondrial activity by measuring the formation of a soluble formazan product which is directly proportional to the number of living cells. For this assay, synovium-derived mesenchymal cells, 106/ml, were seeded in a 24-well tissue culture plate. Cells were seeded onto bone wax and BG/CS/CMC composites in individual wells. Cell-seeded wells without any materials were also prepared as controls. Cells were incubated at 37 °C in 5 % (v/v) CO2. After 3 and 7 days, the medium was discarded. The fresh complete medium was mixed with WST-1 at 10:1 (v/v) and added to the wells and incubated for 2 h at 37 °C in 5 % CO2. After the incubation, 200 μl WST-1 and medium mixture was transferred to a 96-well tissue-culture plate and the absorbance was read at 450 nm. All experiments were carried out in triplicate.
In vivo experiments
The study performed was in accordance with the standards of the guidelines for the care and use of laboratory animals of the NIH (Institute of Laboratory Animal Resources (US) 1996).
Cell-free porous scaffolds of BG/CS/CMC and bone wax were surgically implanted into bone defects for in vivo biodegradation and osteogenesis assessment. Eighteen adult New Zealand rabbits (Experimental Animal Center of Shanghai No.1 Medical University, Shanghai, China), 3–3.5 kg body wt, were used to study bone regeneration and to determine hemostasis potential of BG/CS/CMC composites in femoral condyles. General anesthesia was induced with an intravenous injection of 20 % urethane (4 ml/kg).
The rabbits were placed into three randomized groups. Critical size defects (Giavaresi et al. 2010) (6 mm diam. 10 mm length) were transversally created in the interior of distal femoral condyle of the right posterior limb by a standard surgical procedure in all rabbits. A 4 cm skin incision was made on the interior of the distal femoral condyle. The defects were expanded with a 6 mm drill. The depth of the defects was 10 mm as measured by a digital caliper. Group A (n = 6) was the control group, as nothing was implanted in the defects. Groups B (n = 6) and C (n = 6) were treated with 1 g BG/CS/CMC and 1 g bone wax, respectively. The weight of a BG/CS/CMC composite, 6 mm diam. × 10 mm long) is approx. 1 g. We pressed the implant material into the defects with sterilized gloves.
The bleeding points of the soft tissue surrounding the defects were handled by briefly using an electrotome to reduce variability. Each defect was covered with a piece of sterile sponge with a weight of M1. Ten minutes later, the sponge was removed and weighed as M2. Thus, the weight of errhysis (M3) was obtained by: M3 = M2 − M1. The statistical analysis is described below.
After surgery, three animals in each group were sacrificed with an air injection at 3 and 9 weeks. The distal femoral condyles were taken immediately for X-ray examination. All of the samples were fixed with 4 % paraformaldehyde for histological examination.
X-ray examination
In vivo mineralization and osteogenesis at the rabbit femoral defects repaired with BG/CS/CMC and bone wax were examined with X-rays (Shimadzu, R-30H, CH-200) (W 4096, C 2048) at 3 and 9 weeks post-surgery.
Histological analysis
Histological analysis of the implants was performed. The samples were fixed with 4 % (v/v) paraformaldehyde for 2 weeks, decalcified in 10 % (w/v) EDTA for 3 weeks, embedded in paraffin, and cut into 5 mm thick sections. The sections were stained with hematoxylin, eosin and Masson trichrome staining and then were evaluated under light microscopy. After manually placing the outlines in the bone tissue, the material area of different implants was automatically calculated by ImageJ software using predefined Hounsfield unit thresholds (139–175).
Each section was divided into four quadrants to determine the material remnant fraction. In each quadrant, the remnant material area was defined as the distance between the edge of the bone tunnel and the outer graft determined under 200× magnification. Four separate measurements were made in each of the quadrants, for a total of 16 measurements for each specimen. The average remnant area for each specimen was then determined by averaging the values obtained from each specimen.
Statistics analysis
Statistical analysis was performed using the software package SPSS 13.0 (IBM, ISBN: 9787117070102). Data from the in vivo experiment was analyzed by two-way ANOVA test. Statistical comparisons were carried out using analysis of variance by Origin 7.0 Software (OriginLab, ISBN 9787810773362). A value of p < 0.05 was considered to be statistically significant.
Results and discussion
The development of bone tissue engineering parallels the progress in materials technology. At present, the two main types of bone repair material are characterized as either solid or colloidal. The former is used to repair large bone defects and defects at weight-bearing sites, whereas the latter is mainly applied to non-bearing small bone defects or defects with irregular shape. In this study, 58S bioactive glass was incorporated into CS/CMC matrix to prepare a colloidal BG/CS/CMC composite hydrogel for bone defect hemostasis and bone regeneration applications.
The microstructure of the three-dimensional porous scaffolds of BG/CS/CMC prepared with lyophilization were analyzed with SEM (Fig. 1). High magnification SEM micrographs revealed an interconnected pore structure with congeneric and uniform distribution of bioactive glass particles within the CS/CMC matrices. The average pore size was approx. 78 ± 28 μm. The porosity and pore size play a critical role in the growth of osteoblasts. Pores provide structures on which newly formed bone can be deposited. Our SEM observation showed that the surface morphology of the porous wall was rough. According to previous reports, the surface roughness can enhance attachment, proliferation, and differentiation of anchorage-dependent bone forming cells (Yuan et al. 1999). Bioactive glass particles can be observed on the pore wall surface with good distribution. This structure facilitates bone creeping substitution, which leads to better bone ingrowth compared with ingrowth on a smooth surface.
A WST-1 assay was implemented to quantitatively investigate cell viability of SMSCs cultured with the BG/CS/CMC composite scaffold, bone wax, or alone as a control. As shown in Fig. 2, there was no significant difference in cell viability among the BG/CS/CMC composite scaffold, bone wax, and the control groups after 3 days and after 7 days cultivation. After 7 days of incubation, SMSCs cultured in the three groups had a higher metabolic activity than at 3 days, which we ascribed to the increased proliferation of the SMSCs. The lack of difference among the three groups indicated that both the BG/CS/CMC scaffold and bone wax displayed excellent biocompatibility.
In the in vivo studies, neither hematoma nor infection phenomenon was observed throughout the experiment. In the untreated group, most of the area of the defect was empty and only slight bone formation was found around the periphery of the defects after 9 weeks. In the bone wax group, there was no change in the size of defect throughout the whole experiment. At 9 weeks, the wax still covered the bone defect perfectly (Fig. 3). However, in the BG/CS/CMC group, the diam. of the defect appeared to reduce after 9 weeks after surgery. There was an obvious gap between the remnant composite and the edge of the defect. Moreover, the inner area of the defect had also become occupied by newly formed bone tissue.
Swelling is among the most fundamental properties of hydrogels, and proper swelling of the composite is very useful when implanted into the bone defect. Roman et al. (2008) proposed that this property would allow hydrogels to have tighter contact with surrounding tissues, which prompts hemostasis at the same time. In our study, we evaluated short-term hemostasis quantitatively by determining the weight of errhysis (M3). There was no significant statistic difference (p > 0.05) of the short-term hemostasis between bone wax (M3 = 0.067 ± 0.006) and the BG/CS/CMC group (M3 = 0.074 ± 0.009). Compared with the untreated group (M3 = 0.913 ± 0.032), both groups showed significant hemostasis capability (Fig. 4a). The quantitative results also reflected our direct observation, as we observed very little blood on the sponges recovered from the bone wax and BG/CS/CMC groups.
Figure 5 shows representative X-ray images of the surgical sites for both groups after 9 weeks. The defect in the bone wax group also gradually cicatrized with time, but the amount of newly formed bone was obviously less than in the composite group. In the BG/CS/CMC group, most of the defect was covered with cancellous bone after 9 weeks, and only a small amount of the BG/CS/CMC composite scaffold could be observed. Residual BG/CS/CMC composite was found at the cortical layer of bone (Fig. 5b). In addition, the appearance of the repaired defect was similar to that of the normal femoral condyles.
To detect growth of the bone tissue throughout the entire BG/CS/CMC scaffold, cross sections from the middle of the scaffold were assessed at three and 9 weeks post-surgery with histological observation (only control group). After 3 weeks, the scaffold had markedly degraded into small fragments and failed to maintain its structural integrity, giving way to increased amounts of osteogenic cells and osteoid tissue (Fig. 6A, a). At 9 weeks (Fig. 6B, D), the peripheral of scaffold had been replaced by newly formed bone trabecula, multinuclear giant cells, fiber cells, and capillaries. In residual scaffold areas, there was a large quantity of multinucleate giant cells. The cells appeared in the pores of scaffolds near the surface of the pore walls. This indicated that the multinucleate giant cells play a critical role in scaffold degradation and osteogenesis.
Masson trichrome staining was used for the micrographs of the BG/CS/CMC implants in rabbit bone defects at 9 weeks post-surgery. Green shows newly-formed bone tissue, such as collagen fiber or cartilage. Red shows mature bone tissue, such as calcification of bone and mature collagen. Originally, the composite appeared as a waxy material with few internal pores. We suggest that over time, the implant forms a porous structure via three phases. First, the moisture content is extruded out of the implant. Second, the composite electrolytes gradually depolymerized through charge exchange. Third, the composite degrades, with ongoing pore formation and cell ingrowth.
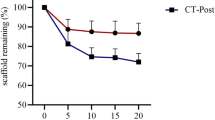
Ideally, in the repair process, the scaffold should be gradually absorbed or metabolized in vivo. Thus, it is important to regulate the balance between scaffold degradation and new bone formation. The in vitro degradation rate of chitosan is rather slow but the combination of CMC with chitosan can greatly improve the degradation rate of chitosan (Jiang et al. 2013). When CMC and chitosan had degraded, bioactive glass promoted the process of bone regeneration. The initial proportion of CMC was approx. 65 %; thus, the BG/CS/CMC scaffold degraded quickly in the first 3 weeks. During the process, inorganic phosphate and Ca2+ were gradually released into the surrounding environment to aid bone reconstruction (Kim et al. 2005). However, compared with the histological appearance at 3 weeks, the remnant material at 9 weeks tended to form smaller fragments and concentrated in the center of the implant. The average remnant area of the BG/CS/CMC scaffold decreased from 29 to 18 % (Fig. 4b). The degradation rate of the composite can be controlled by changing its molecular weight.
Conclusions
The short-term hemostatic effect of a BG/CS/CMC composite in the bone defect showed no statistically significant differences compared with conventional bone wax. Biodegradation and new bone formation processes linked up very well. Furthermore, the composite had no cytotoxicity while segmental bone defects were healed with new-formed bone within 9 weeks of implantation. Thus, BG/CS/CMC composite could be a satisfying alternative for bone wax.
References
Cho SK, Yi JS, Park MS, Hu G, Zebala LP, Pahys JM, Kang MM, Lee DH, Riew KD (2012) Hemostatic techniques reduce hospital stay following multilevel posterior cervical spine surgery[J]. J Bone Joint Surg 94(21):1952–1958
De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP (2001) Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum 44:1928–1942
Giavaresi G, Fini M, Salvage J, Nicoli AN, Giardino R, Ambrosio L, Nicolais L, Santin M (2010) Bone regeneration potential of a soybean-based filler: experimental study in a rabbit cancellous bone defects. J Mater Sci Mater Med 21(2):615–626
Gibbs L, Kakis A, Weinstein P, Conte JE Jr (2004) Bone wax as a risk factor for surgical-site infection following neurospinal surgery[J]. Infect Control Hosp Epidemiol 25(4):346–348
Institute of Laboratory Animal Resources (US) (1996) Guide for the care and use of laboratory animals[M]. National Academies Press, Washington
Jiang H, Zuo Y, Zou Q, Wang H, Du J, Li Y, Yang X (2013) Biomimetic spiral–cylindrical scaffold based on hybrid chitosan/cellulose/nano-hydroxyapatite membrane for bone regeneration. ACS Appl Mater Interfaces 5:12036–12044
Kim HW, Kim HE, Salih V (2005) Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin-hydroxyapatite for tissue engineering scaffolds. Biomaterials 26:5221–5230
Lew KS, Othman R, Ishikawa K, Yeoh FY (2012) Macroporous bioceramics: a remarkable material for bone regeneration. J Biomater Appl 27(3):345–358
Roman J, Cabanas MV, Pena J, Doadrio JC, Vallet-Regi M (2008) An optimized beta-tricalcium phosphate and agarose scaffold fabrication technique. J Biomed Mater Res A 84:99–107
Shi J, Alves NM, Mano JF (2007) Thermally responsive biomineralization on biodegradable substrates[J]. Adv Funct Mater 17(16):3312–3318
Vestergaard RF, Jensen H, Vind-Kezunovic S, Jakobsen T, Søballe K, Hasenkam JM (2010) Bone healing after median sternotomy: a comparison of two hemostatic devices. J Cardiothorac Surg 24(5):117–123
Walsh WR, Morberg P, Yu Y, Yang JL, Haggard W, Sheath PC, Svehla M, Bruce WJ (2003) Response of a calcium sulfate bone graft substitute in a confined cancellous defect. Clin Orthop Relat Res 2:228–236
Yuan H, Kurashina K, de Bruijn JD, Li Y, de Groot K, Zhang X (1999) A preliminary study on osteoinduction of two kinds of calcium phosphate ceramics. Biomaterials 20:1799–1806
Zhang X, Zhu L, Lv H, Cao Y, Liu Y, Xu Y, Ye W, Wang J (2012) Repair of rabbit femoral condyle bone defects with injectable nanohydroxyapatite/chitosan composites. J Mater Sci 23:1941–1949
Acknowledgments
The authors thank the financial support of the National Natural Science Foundation of China (Grant No. 81071468) and Shanghai Municipal Science and Technology Commission Medical Guide Project (Grant No. 124119a7500).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, C., Li, H., Pan, J. et al. Biodegradable composite scaffolds of bioactive glass/chitosan/carboxymethyl cellulose for hemostatic and bone regeneration. Biotechnol Lett 37, 457–465 (2015). https://doi.org/10.1007/s10529-014-1697-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1697-9