Abstract
We have explored the feasibility of using adipose-derived stem cells (ADSCs) and polyglycolic acid (PGA) for constructing muscular tubes of urethra in a bioreactor. With the induction of by 5-azacytidine, ADSCs were found to acquire a myoblast phenotype. Here we seeded ADSCs in a PGA mesh to construct the cell–PGA complex that was cultured statically for 1 week. Afterwards, the cell–PGA complex was subjected to extension stimulation in a bioreactor for 5 weeks. A muscular tube of urethra was formed after 6 weeks. Histological examination showed differentiated ADSCs and collagenous fibers had orientated well. This study demonstrates that tissue engineering of urethra tissues in vitro by using a bioreactor leads to tissue maturation and the differentiation of ADSCs. This novel technique could provide an effective approach for urethra tissue engineering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urethral defects resulting from urinary injury, tumor resection, infection or malformation are a disturbing urological disease. Current surgical repair techniques require autogenous tissue, such as genital skin (Rehder et al. 2010; Mathur et al. 2007), bladder mucosa (Marzorati et al. 2008), or oral mucosa (Haque et al. 2012; Andrich and Mundy 2001), to reconstruct such defects. However, the limited supply of donor materials has greatly confined their use. Moreover, the repair of urethral defects often leads to various complications such as stricture, lithogenesis, hair growth, and diverticula formation. Tissue engineering is an approach for obtaining satisfactory urethral tissue that could circumvent shortcomings of current available options. The production of a two-layered engineered urethra using oral epithelial and muscle-derived cells has been reported (Mikami et al. 2012). Moreover, reconstructive engineered urethras with a well-developed muscle layer represent a good solution to avoid urethral strictures. However, mature muscle cells, such as smooth muscle cells (SMCs) or myoblasts, are difficult to isolate and have limited ability of proliferation. These disadvantages make it necessary to explore a suitable cell source for urethral engineering.
Mesenchymal stem cells (MSCs) have drawn attention because they are able to self-renew with a high proliferative capacity and have a potential to differentiate into diverse cell types (Barry and Murphy 2004; Pittenger et al. 1999). During the recruitment of these cells, the use of adipose-derived stem cells (ADSCs) is favored because ADSCs are easier to acquire, and have relatively less donor site damage (Mizuno et al. 2012; Zhu et al. 2008; Rodríguez et al. 2006). ADSCs are a promising source of myoblasts, as chemical stimulation can trigger their differentiation into myoblasts (Fu et al. 2010). Myoblasts could be influenced by the surrounding tissue environment and muscle cells, and these influences could turn myoblasts into SMCs types. Accumulating evidence has demonstrated that mechanical stimulation is important for improving the biomechanical function of tissues such as cartilage, bone, muscle and smooth muscle (Bian et al. 2010; Benhardt and Cosgriff-Hernandez 2009). Mechanical stimulation is also effective in initiating the differentiation of ADSCs into functional muscle tissue (Dado et al. 2012; Lee et al. 2007; Marra et al. 2011).
In the present study, we have isolated and cultured ADSCs in vitro and seeded undifferentiated ADSCs onto polyglycolic acid (PGA) scaffolds to explore the feasibility of constructing a muscular tube of urethra in a bioreactor system.
Materials and methods
Isolation and culture of canine adipose-derived stem cells (ADSC) in vitro
Adipose tissue was obtained from the inguinal regions of adult beagle dogs. The experimental protocol was approved by the Research Ethical Committee of the Hospital. The isolation and culture of ADSCs were performed as previously described (Zuk et al. 2002; Arrigoni et al. 2009). Briefly, after washing in phosphate-buffered saline (PBS) three times, the fresh adipose tissues were cut into small pieces, and then digested with 0.10 % collagenase I (Sigma) with shaking at 37 °C for 1 h. After digestion, the collagenase I was neutralized with low-glucose Dulbecco’s modified Eagle’s medium (LG-DMEM) supplemented with 10 % (v/v) fetal bovine serum (FBS) and the suspension was further filtered with a 50 μm mesh filter to remove the undigested tissue and then centrifuged at 1,500×g for 5 min. The cells were re-suspended in LG-DMEM supplemented with 10 % (v/v) FBS, 100 U penicillin/ml and 100 μg streptomycin/ml (defined as the normal medium) and cultured at 37 °C with 95 % humidity and 5 % CO2. These cultured cells were referred as P0 cells. The characterization of ADSCs was determined by their CD marker profile. After culturing for 3–5 days, the cell colonies reached 70–80 % confluence and were then passaged with trypsin/EDTA. ADSCs of passage 1 were used for the study.
Induction of ADSCs into myoblasts
After P0, ADSCs were digested with trypsin then 10 μM 5-Aza (5-azacytidine, Sigma-Aldrich), 5 % (v/v) FBS and 5 % (v/v) horse serum (Gibco) were added into LG-DMEM (defined as the inducing medium), The induction of ADSCs were performed for 4 weeks as described by Fu et al. (2010). Cell growth and morphology were constantly monitored by inverted microscopy. Immunofluorescent staining with the myoblast-specific markers, desmin and α-SMA, was carried out in both induced and untreated cells. Canine bladder smooth muscle cells (BSMC) were used as positive controls.
Preparation of polyglycolic acid (PGA) tubing scaffold
PGA unwoven fibers (Albany International Research, Mansfield, USA), 15 μm diameter.; 40 mg wt, were made into a 20 × 15 × 1 mm mesh. The PGA mesh was sewn around medical-grade silicone tube and then the tubing scaffold was processed before use as described by Wang et al. (2010). Scaffolds were disinfected with 75 % (v/v) ethanol for 2 h and then briefly washed three times with PBS. We then incubated the tubing scaffold with LG-DMEM (containing 10 % FBS, penicillin/streptomycin) overnight to enhance cell attachment. The medium was removed afterward and the scaffold was air-dried for 45 min under UV before use.
ADSC seeding onto PGA meshes
ADSCs at passage 1 were collected, re-suspended in the culture medium at 6 × 107 cells/ml, and 4 × 107cells were seeded by instillation into each PGA mesh in tissue culture dishes. Thereafter, the cell–PGA complexes were kept in an incubator for 4 h to allow the cells to adhere onto the PGA fibers. During this, the complexes were rotated 90° around a longitudinal axis every 15 min to promote homogeneous cell adhesion in the scaffolds. Finally, the DMEM with 10 % (v/v) FBS was added to cover the cell–PGA constructs. The constructs were incubated for another 7 days in the culture dishes. Afterwards some of the samples were examined by scanning electron microscope while others were transferred to a bioreactor for further incubation.
Pulsatile flow bioreactor culture
To conduct mechanical extension stimulation, we first designed and manufactured a bioreactor that was a closed-loop perfusion system, consisting of a reaction chamber, a peristaltic pump, a fluid collection bottle and connection ducts (Fig. 3). The mechanical extension was rhythmical and was produced by a peristaltic pump at 75 times/min, with a pressure of 0.02 MPa. After 7 days of cultivation in dishes, the ADSCs-seeded tubular PGA scaffolds was threaded through the side arms of the chamber and secured. The culture period included a 1 week proliferation phase and a 4 weeks differentiation phase. The cell–PGA constructs were incubated in LG-DMEM (containing 10 % FBS, penicillin/streptomycin) during the first week of culture. After 1 week, the culture medium was replaced by the inducing medium. The circulating culture medium was replaced once every 7 days.
Analysis of cell-seeded scaffolds
After 6 weeks of culture, ADSCs-seeded tubular PGA scaffolds were rinsed with PBS, fixed with 4 % (v/v) paraformaldehyde for 24 h and embedded in paraffin wax. The specimens were cut into 4 μm thick sections. HE staining and Masson staining for collagen were carried out. Formation of myoblasts was evaluated by immunohistochemical staining. The tissue sections were stained using antibodies against desmin (1:100, Abcam, Cambridge, UK), and α-SMA (1:100, Abcam, Cambridge, UK), followed by an addition of goat anti-mouse IgG conjugated with horseradish peroxidase (EnVision + System, Dako). Finally, the color was developed with Liquid DAB + Substrate Chromogen System (Dako K3467, Dako Corp.) before counterstaining with hematoxylin.
Results
Characterization of ADSCs
Phase-contrast microscopy revealed that, when isolated from donor adipose tissue and cultured as a monolayer, ADSCs began to be attached after 24 h and reached 80 % confluence after 4–5 days. Most of the adhering ADSCs exhibited a fibroblast-like spindle shape (Fig. 1a, b). Uniform cell morphology and clustered cell growth were also observed. The ADSCs were assessed by flow cytometric analysis. High expression of MSC markers CD90, CD44 and CD105 was found in ADSCs. In contrast, CD34 and CD45 were lowly expressed (Fig. 1c).
Differentiation of ADSCs towards myoblasts
Both a microscopic observation and an immunofluorescence analysis were performed to assess the differentiation of ADSCs towards myoblasts after induction. As shown in Fig. 2, induced myoblasts grew in a ‘‘hill and valley’’-like pattern similar to that observed in isolated BSMCs. When treated with 5-Aza, the expression of desmin and α-SMA could be detected in induced myoblasts. As a positive control, expression of the myoblast markers mentioned above was examined in BSMCs. Weak α-SMA expression and no expression of desmin could be detected in untreated ADSCs as a negative control.
a, d, g ADSCs treated with basal medium only as a control. b, e, h ADSCs exhibit a “hill and valley”-like pattern similar to that of BSMCs after treatment in basal medium supplemented with 5-Aza for 4 weeks. c, f, i Canine bladder smooth muscle cells (BSMCs) as a positive control. Immunofluorescent staining (green) was performed for α-SMA and desmin at 4 weeks. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Bar scales 100 μm
Culture of ADSCs–PGA complex
ADSCs collected from passage 1 were seeded on the PGA scaffold (Fig. 3a). After being incubated for 24 h, ADSCs began to adhere and spread along the length of the PGA fibers and the cell shape changed from round to an extended form. The cell–PGA constructs were maintained in the normal medium for 7 days to allow the cells to deposit extracellular matrix in the scaffold. As a result, SEM observations showed that extracellular matrices filled the space between the PGA fibers (Fig. 3).
Culture of ADSCs–PGA complex in bioreactor
We observed the effects of mechanical extension in myoblasts induction. After isolating ADSCs, we assembled them into a tabularized bioabsorbable PGA scaffold. After being incubated in a dish for 7 days, the ADSCs–PGA constructs were then incubated in a reaction chamber of a bioreactor for 5 weeks. We produced a glossy tubular structure with a 6 mm lumen from the mechanical extension-stimulated group (Fig 4a, b).
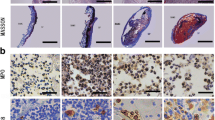
a, b Gross view of tissue-engineered muscular tubes of urethra after dynamic cultured for 6 weeks. The tube diameter is ~6 mm. c Hematoxylin and eosin staining shows that PGA fibers are nearly degraded completely in the dynamic group. d Masson staining shows more collage fibers distributed evenly in the dynamic group. Many myoblast-like cells expressed desmin (e) and α-SMA (f) in the middle layer of engineered muscular tubes of urethra cultivated with mechanical extension stimulation
Histological evaluation of tissue microstructure
Using HE, Masson and immunohistochemical staining analyses, we discovered that the PGA scaffold adsorbed more quickly in extension-induced muscular tubes and these tubes exhibited increased expression of collagen fibers, desmin and α-smooth muscle actin (α-SMA) (Fig. 4). Desmin and α-SMA are markers of myoblasts, and collagen fibers are the main structural constituents of urethras. Therefore, our results showed that mechanical extension combined with chemical induction can generate myoblasts from ADSCs.
Discussion
Despite accumulating evidence showing that urethra engineering holds great promise in treating urethral defects, cell source remains a major obstacle in the progress of urethra engineering. Some investigators have used SMCs as seed cells for muscular urethral construction (Mikami et al. 2012) but acquiring enough seed SMCs is difficult because of their low proliferation potential and relatively higher trauma during primary cell harvesting. ADSCs have a higher proliferation potential compared to SMCs and are relatively easier to obtain; therefore, we used ADSCs to differentiate into myoblasts. This approach facilitates the easy procurement of enough seed cells and reduces the injury to donor sites.
In the present study, the ADSCs were harvested from canine inguinal adipose tissues. Flow cytometric analysis showed that the ADSCs were positive for CD90, CD44 and CD105, but negative for CD34 and CD45, which is consistent with previous reports (Mitchell et al. 2006; Liao et al. 2008; Ahn et al. 2008) and suggests that these cells have a mesenchymal origin. According to Wakitani et al. (1995), 5-Aza can induce stroma stem cells from bone marrow to differentiate into myoblasts. Similar results were obtained by Fu et al. (2010) using ADSCs, Based on these studies, we demonstrated that ADSCs acquired the phenotype of myoblasts as evidenced by their expression of specific structural proteins including α-SMA, and desmin, upon induction with 5-Aza in vitro. Though ADSCs have differentiated into myoblasts by 5-azacytidine stimulation, the proliferative ability and extracellular matrices production become lower than undifferentiated ADSCs. Hence, we constructed a muscular conduit of urinary tract using the undifferentiated ADSCs and PGA fiber mesh in a bioreactor system.
PGA is one of the most popular biodegradable scaffolds and has been used in tissue engineering because of its good biocompatibility and suitable degradation rate (Niklason et al. 2001). In the present study only a few remnant undegraded PGA fibers could be observed histologically in the dynamic group, which shows that mechanical extension might render a positive effect on degradation of PGA thereby avoiding inflammation after implantation of tissue-engineered urethras (Gui et al. 2011). The mechanical ability of urethra graft to resist the washing-out effect of urinary flow is critically important to long-term clinical use. The ultimate mechanical properties of tissues are generally associated with collagen density (Niklason et al. 1999). Moreover, the tissue-engineered urethras generated in dynamic environment, exhibited well-differentiated myoblasts and organized collagen fibers, indicating that mechanical extension may further facilitate remodeling of tissue-engineered urethras.
These results confirm the important role of cyclic extension in the development of urethra tissue engineering and suggest that ADSCs in tissues engineered under mechanically active conditions retain a differentiated myoblast phenotype upon induction with 5-Aza. These in vitro findings of excellent cellular penetration and collagen deposition within the PGA support the feasibility of the cell–PGA complex under the dynamic culture conditions for applications in urethra tissue engineering.
In our present study, we have only constructed a muscular layer of urethra, which is different from native urethras and, although the bioreactor provides mechanical extension in constructing engineered urethras, we only used one extension strength which is not enough to determine the optimal mechanical stimulation mode. Therefore, further studies are ongoing to seed epithelial cells on lumen surfaces and implant them in vivo to repair canine urethral defects.
In conclusion, we demonstrate that ADSCs can differentiate into myoblasts when induced with 5-Aza, and mechanical extension stimulation can improve the viability of ADSCs induced to differentiate into myoblasts in vitro. Our findings indicate that mechanical extension stimulation may further facilitate remodeling of engineered muscular tubes of urethra.
References
Ahn HH, Lee JH, Kim KS, Lee JY, Kim MS et al (2008) Polyethyleneimine-mediated gene delivery into human adipose derived stem cells. Biomaterials 29:2415–2422
Andrich DE, Mundy AR (2001) Substitution urethroplasty with buccal mucosal-free grafts. J Urol 165:1131–1134
Arrigoni E, Lopa S, de Girolamo L, Stanco D, Brini AT (2009) Isolation, characterization and osteogenic differentiation of adipose-derived stem cells: from small to large animal models. Cell Tissue Res 338:401–411
Barry FP, Murphy JM (2004) Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol 36:568–584
Benhardt HA, Cosgriff-Hernandez EM (2009) The role of mechanical loading in ligament tissue engineering. Tissue Eng B 15:467–475
Bian L, Fong JV, Lima EG, Stoker AM, Ateshian GA et al (2010) Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng A 16:1781–1790
Chancellor MB, Yokoyama T, Tirney S, Mattes CE, Ozawa H et al (2000) Preliminary results of myoblast injection into the urethra and bladder wall: a possible method for the treatment of stress urinary incontinence and impaired detrusor contractility. Neurourol Urodyn 19:279–287
Dado D, Sagi M, Levenberg S, Zemel A (2012) Mechanical control of stem cell differentiation. Regen Med 7:101–116
Fu Q, Song XF, Liao GL, Deng CL, Cui L (2010) Myoblasts differentiated from adipose-derived stem cells to treat stress urinary incontinence. Urology 75:718–723
Gui L, Zhao L, Spencer RW, Burghouwt A, Taylor MS et al (2011) Development of novel biodegradable polymer scaffolds for vascular tissue engineering. Tissue Eng A 17:1191–1200
Haque ME, Rahman MA, Islam MF, Siddique FH, Uddin MM et al (2012) Ventral free oral mucous membrane graft for bulbar urethral stricture. Mymensingh Med J 21:696–701
Lee WC, Maul TM, Vorp DA, Rubin JP, Marra KG (2007) Effects of uniaxial cyclic strain on adipose-derived stem cell morphology, proliferation, and differentiation. Biomech Model Mechanobiol 6:265–273
Liao X, Li F, Wang X, Yanoso J, Niyibizi C (2008) Distribution of murine adipose-derived mesenchymal stem cells in vivo following transplantation in developing mice. Stem Cells Dev 17:303–314
Marra KG, Brayfield CA, Rubin JP (2011) Adipose stem cell differentiation into smooth muscle cells. Methods Mol Biol 702:261–268
Marzorati G, Ghinolfi G, Pachera F, Meazza A (2008) Bladder and buccal mucosa graft in urethral stricture reconstruction. Urologia 75:177–179
Mathur RK, Himanshu A, Sudarshan O (2007) Technique of anterior urethra urethroplasty using tunica albuginea of corpora cavernosa. Int J Urol 14:209–213
Mikami H, Kuwahara G, Nakamura N, Yamato M, Tanaka M et al (2012) Two-layer tissue engineered urethra using oral epithelial and muscle derived cells. J Urol 187:1882–1889
Mitchell JB, Mcintosh K, Zvonic S, Garrett S, Floyd ZE et al (2006) Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 24:376–385
Mizuno H, Tobita M, Uysal AC (2012) Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 30:804–810
Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S et al (1999) Functional arteries grown in vitro. Science 284:489–493
Niklason LE, Abbott W, Gao J, Klagges B, Hirschi KK et al (2001) Morphologic and mechanical characteristics of engineered bovine arteries. J Vasc Surg 33:628–638
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Rehder P, Glodny B, Pichler R, Exeli L, Kerschbaumer A et al (2010) Dorsal urethroplasty with labia minora skin graft for female urethral strictures. BJU Int 106:1211–1214
Rodríguez LV, Alfonso Z, Zhang R, Leung J, Wu B et al (2006) Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci USA 103:12167–12172
Wakitani S, Saito T, Caplan AI (1995) Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve 18:1417–1426
Wang C, Cen L, Yin S, Liu Q, Liu W et al (2010) A small diameter elastic blood vessel wall prepared under pulsatile conditions from polyglycolic acid mesh and smooth muscle cells differentiated from adipose-derived stem cells. Biomaterials 31:621–630
Zhu Y, Liu T, Song K, Fan X, Ma X et al (2008) Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct 26:664–675
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (30973016) and Shanghai Natural Science Foundation (09ZR1424100).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Fu, Q., Zhao, RY. et al. Muscular tubes of urethra engineered from adipose-derived stem cells and polyglycolic acid mesh in a bioreactor. Biotechnol Lett 36, 1909–1916 (2014). https://doi.org/10.1007/s10529-014-1554-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1554-x