Abstract
β-Glucosidase from Thermus thermophilus has specific hydrolytic activity for the outer glucose at the C-20 position in protopanaxadiol-type ginsenosides without hydrolysis of the inner glucose. The hydrolytic activity of the enzyme for gypenoside XVII was optimal at pH 6.5 and 90 °C, with a half-life of 1 h with 3 g enzyme l−1 and 4 g gypenoside XVII l−1. Under the optimized conditions, the enzyme converted the substrate gypenoside XVII to ginsenoside F2 with a molar yield of 100 % and a productivity of 4 g l−1 h−1. The conversion yield and productivity of ginsenoside F2 are the highest reported thus far among enzymatic transformations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ginseng (the roots of Panax ginseng C. A. Meyer) is used as a traditional medicine in Asian countries and ginsenosides in ginseng exhibit important biological activities, such as anticancer (Lee et al. 2009), antifatigue (Yoshikawa et al. 2003), antiallergic (Bae et al. 2002), anti-inflammatory (Wang et al. 2011b), and antioxidant (Cho et al. 2006) activities. Ginsenoside F2 especially exhibits anticancer effects against glioblastoma multiforme (Shin et al. 2012). The minor ginsenosides, including F2, Rg3, Rh2, compound K, compound Mc, compound Y, and aglycone protopanaxadiol, which are absent or present at low concentrations in ginseng, exert greater pharmacological effects than the major ginsenosides, including Rb1, Rb2, Rc, and Rd (Xu et al. 2003). Therefore, many studies have focused on the conversion of the major ginsenosides to the minor ginsenosides by the hydrolysis of the sugar moieties of ginsenosides.
Ginsenoside F2 has been converted from Rb2 by crude extracts of Caulobacter leidyia (Cheng et al. 2006) and Intrasporangium sp. (Cheng et al. 2007), from Rb1 by crude extracts of Leuconostoc mesenteroides (Quan et al. 2011) and Lactobacillus paralimentarius (Quan et al. 2013), from gypenoside XVII by β-glucosidase from Flavobacterium johnsoniae (Hong et al. 2012), from Rd, gypenoside XVII, and compound Mc1 by β-glucosidase from Sphingomonas sp. (Wang et al. 2011a), and from Rd and gypenoside XVII by β-glucosidase from Pythium irregulare (Andreea Neculai et al. 2009). β-Glucosidase from F. johnsoniae specifically hydrolyzes the outer glucose linked to the C-20 position in the protopanaxadiol (PPD)-type ginsenosides Rb1 and gypenoside XVII. However, the substrate specificity of this enzyme has not been investigated. Moreover, the quantitative production of F2 from diverse ginsenosides has not been attempted.
In this study, among the cloned β-glucosidases, a thermostable β-glucosidase from Thermus thermophilus was found to have highly selective hydrolytic activity for the outer glucose at the C-20 position in PPD-type ginsenosides, and the enzyme was applied to the quantitative production of ginsenoside F2 from gypenoside XVII.
Materials and methods
Materials
The ginsenoside standards F2, R1, R2, Rb2, Rc, Rd, Re, Rg1, Rg2, Rg3, Rf, Rh1, Rh2, and compound K were purchased from Sigma, BTGin (Daejeon, Korea), and Ambo Laboratories (Daejeon, Korea). Gypenoside XVII and LXXV were prepared from Rb1 and gypenoside XVII, respectively, by β-glucosidases from Sphingopyxis alaskensis (Shin and Oh 2013) and Dictyoglomus turgidum (Lee et al. 2012), respectively. The reactions were performed at 50 and 80 °C, respectively, in 50 mM phosphate/citrate buffer (pH 5.5) containing 1 mM ginsenoside and 0.5 mg enzyme l−1 for 10 h. The product solutions were then mixed with Celite 545 (Daejung, Shiheung, Korea) to adsorb the products. The solutions were filtered through Whatman filter paper No. 2, and the solid particles were eluted with 100 % methanol at 60 °C for 1 h using a sonicator. The eluted solutions were dried using a centrifugal evaporator and the dried materials were used as ginsenoside standards and substrates.
Bacterial strains, plasmid, and culture conditions
Genomic DNA from T. thermophilus DSMZ 579, Escherichia coli ER2566, and pET-24a(+) were used as the sources of β-glucosidase gene, host cells, and expression vector, respectively. Recombinant E. coli for protein expression was cultivated with shaking at 200 rpm in a 2 l Erlenmeyer flask containing 500 ml LB medium at 37 °C with 20 μg kanamycin ml−1 until the OD600 reached 0.6. IPTG was then added at 0.1 mM to induce enzyme expression. The culture was grown at 16 h with shaking at 150 rpm and 16 °C.
Gene cloning
The β-glucosidase gene (1,296 bp) was amplified by PCR using genomic DNA isolated from T. thermophilus as a template. Primer sequences were based on the DNA sequence of a glycosyl hydrolase 1 family domain protein from T. thermophilus (GenBank accession number YP_145326.1). Forward (5′-CATATGATGACCGAGAACGCCGAAAAATT-3′) and reverse primers (5′-AAGCTTTTTAGGTCTGGGCCCGCGCGA-3′) were designed to introduce the NdeI and HindIII restriction sites (underlined), respectively. The PCR product was subcloned into the pET-24a(+) plasmid digested with the same restriction enzymes and then transformed into E. coli ER2566.
Enzyme purification
Cells were harvested from culture broth and disrupted on ice using a sonicator in 50 mM phosphate buffer (pH 7.0) containing 300 mM NaCl and 1 mg lysozyme ml−1. Unbroken cells and cell debris were removed by centrifugation at 13,000×g for 20 min at 4 °C, and the supernatant obtained was applied to a His-trap affinity chromatography column equilibrated with 50 mM phosphate buffer (pH 7.0). The bound protein was subsequently eluted at 4 °C with the same buffer containing 250 mM imidazole at 1 ml min−1. The active fractions were collected and dialyzed at 4 °C for 16 h against 50 mM citrate/phosphate buffer (pH 5.5). The resulting solution was used as the purified enzyme. The purification step using the column was conducted using a FPLC system at 4 °C.
Hydrolytic activity
One unit (U) of enzyme activity used with aryl-glycoside or ginsenoside was defined as the amount of enzyme required to liberate 1 μmol p-nitrophenol (pNP) or ginsenoside F2 from pNP-β-d-glucopyranoside or gypenoside XVII as a substrate per min at 90 °C and pH 6.5, respectively. The hydrolytic reactions were performed at 90 °C for 10 min in 50 mM phosphate/citrate buffer (pH 6.5) containing 1 mM aryl-glycoside and 24.7 U enzyme l−1, or containing 0.5 mM ginsenoside and 8.1 U enzyme l−1. The activity for aryl-glycosides was determined by the increase in absorbance 450 nm due to the release of NP. The activity for ginsenoside was measured from the increase in the product ginsenosides.
Analytical methods
A reaction solution containing digoxin as an internal standard was extracted with an equal volume of n-butanol. The n-butanol fraction was evaporated to dryness and methanol was added (Huang et al. 2006). Ginsenosides were assayed by HPLC at 203 nm with a C18 column. The column was eluted at 37 °C with acetonitrile/water from 20:80 (v/v) to 80:20 (v/v) at 1 ml min−1.
Results and discussion
Substrate specificity of β-glucosidase from T. thermophilus for aryl-glycosides and ginsenosides
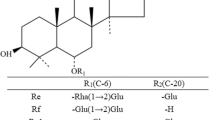
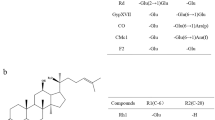
The substrate specificity of β-glucosidase from T. thermophilus was investigated using aryl-glycoside and ginsenosides. The specific activity for aryl glycosides followed the order pNP-β-d-glucopyranoside > pNP-β-d-galactopyranoside > oNP-β-d-glucopyranoside > oNP-β-d-xylopyranoside > pNP-β-d-mannopyranoside > pNP-α-l-arabinopyranoside > pNP-β-d-xylopyranoside. However, no activity for pNP-α-d-glucopyranoside, pNP-α-d-galactopyranoside, pNP-α-l-arabinofuranoside, and pNP-α-l-rhamnopyranoside was found (Table 1). The enzyme exhibited hydrolytic activity for β-1,4-linked and β-1,2-linked aryl-glycosides, whereas it showed no activity for α-1,4 linked aryl-glycosides except for pNP-α-l-arabinopyranoside. The specific activity for ginsenosides as the substrates was in the order Rb1 > gypenoside XVII > gypenoside LXXV (Table 1). No activity was observed for other ginsenosides, including Rd, F2, compound K, R1, R2, Rb2, Rc, Re, Rg1, Rg2, Rg3, Rf, Rh1, and Rh2.
The ginsenoside standards and reaction products were analyzed by HPLC using the C18 column. The ginsenoside standards Rb1, Rd, Rg3, gypenoside XVII, F2, Rh2, gypenoside LXXV, compound K, and APPD were detected based on retention times of 9.5, 12.4, 21.2, 13.9, 18.7, 28.7, 18.2, 27.5, and 32.0 min, respectively (Fig. 1). The products obtained from Rb1, gypenoside XVII, and gypenoside LXXV by β-glucosidase from T. thermophilus were identified as Rd, F2, and compound K, respectively, based on retention times, which were the same as those of the ginsenoside standards, with no formation of the byproducts Rg3, Rh2, and APPD, respectively. Moreover, the reaction products of Rd, F2, and compound K were not further hydrolyzed by the enzyme. Thus, Rb1, gypenoside XVII, and gypenoside LXXV were converted to Rd, F2, and compound K, respectively, which were not further hydrolyzed by the enzyme (Fig. 2). T. thermophilus β-glucosidase hydrolyzes only the outer glucose linked to the C-20 position in PPD-type ginsenosides with a β-1,6 linkage and does not hydrolyze the inner glucose linked to the C-20 position, the other outer glycosides (arabinopyranose and arabinofuranose) linked to the glucose at the C-20 position, or the inner glucose and outer glycosides (xylose, rhamnose, and glucose) linked to the C-3 and C-6 positions. The ginsenoside F2 producing β-glucosidases from Sphingomonas sp. (Wang et al. 2011a) and P. irregulare (Andreea Neculai et al. 2009) hydrolyzed both the outer glucoses linked to the C-3 and C-20 positions in PPD-type ginsenosides. β-Glucosidase from F. johnsoniae (Hong et al. 2012) converted Rb1 and gypenoside XVII to Rd and F2, respectively, by hydrolyzing the outer glucose at the C-20 position. However, the detailed substrate specificity of this enzyme for ginsenosides has not been investigated. These results suggested that T. thermophilus β-glucosidase exhibits novel narrow substrate specificity.
HPLC profiles for the conversions of ginsenosides by β-glucosidase from T. thermophilus. a Conversion of ginsenoside Rb1 to Rd in 30 min and 36 h and the ginsenoside standards Rb1 (retention time, 9.5 min), Rd (12.4 min), and Rg3 (21.2 min). b Conversion of gypenoside XVII to ginsenoside F2 in 30 min and 36 h and the ginsenoside standards gypenoside XVII (13.9 min), F2 (18.7 min), and Rh2 (28.7 min). c Conversion of gypenoside LXXV to compound K in 30 min and 36 h and the ginsenoside standards gypenoside LXXV (18.2 min), compound K (27.5 min), and APPD (32.0 min)
Effects of pH and temperature on the activity of β-glucosidase from T. thermophilus for gypenoside XVII
The product, Rd, which was converted from Rb1 by the reaction of T. thermophilus β-glucosidase, was a major ginsenoside, whereas F2, which was converted from gypenoside XVII, was a minor ginsenoside. Therefore, gypenoside XVII as a substrate was used for the enhanced production of F2. No ginsenoside F2 was formed when the reactions were performed with gypenoside XVII in the absence of enzyme or in the presence of E. coli cells, which lack the β-glucosidase gene from T. thermophilus. The maximum hydrolytic activity of β-glucosidase from the thermophile T. thermophilus for the production of F2 from gypenoside XVII was observed at pH 6.5 and 90 °C (data not shown). The activity of β-glucosidase from F. johnsoniae for gypenoside XVII that was converted to F2 (Hong et al. 2012) was optimal at pH 6.0 and 37 °C. β-Glucosidase from T. thermophilus displayed first-order kinetics for thermal inactivation, and the half-lives of the enzyme at 70, 75, 80, 85, and 90 °C were 437, 125, 70, 10, and 1 h, respectively (Fig. 3). β-Glucosidase from T. thermophilus was the most thermostable enzyme among β-glucosidases that produce ginsenoside F2 from diverse ginsenosides (Neculai et al. 2009; Wang et al. 2011a; Hong et al. 2012).
Thermal inactivation of the activity of β-glucosidase from T. thermophilus for ginsenoside Rd. The enzyme was incubated at 70 (filled triangle), 75 (open square), 80 (filled square), 85 (open circle), and 90 °C (filled circle) in 50 mM phosphate/citrate buffer (pH 6.5) for various periods of time. A sample was withdrawn at each time point and assayed in 50 mM citrate/phosphate buffer (pH 6.5) containing 0.5 mM gypenoside XVII and 8.1 U enzyme l−1 at 90 °C for 20 min. Data represent the means of three experiments, and error bars represent the standard deviation. The relative activity of 100 % was 0.16 mM F2
Production of ginsenoside F2 from gypenoside XVII by β-glucosidase from T. thermophilus
The effect of enzyme concentration on F2 production was investigated with 2 g gypenoside XVII l−1 as the substrate by varying the enzyme concentration from 0.1 (0.16) to 4 g l−1 (6.4 U ml−1) after 20 min. F2 production increased with increasing the enzyme concentrations up to 3 g enzyme l−1 (4.8 U ml−1). However, above 3 g l−1, gypenoside XVII was completely converted to F2 (Fig. 4a), indicating that the enzyme concentration was optimal at 3 g l−1. The production of F2 from gypenoside XVII was assessed with 3 g enzyme l−1 for 20 min by varying the concentration of gypenoside XVII from 0.5 to 10.0 g l−1. Up to 2 g l−1 gypenoside XVII, the conversion yield of gypenoside XVII to F2 was constant. However, above 2 g l−1, the conversion yield decreased. F2 production increased with increasing the concentration of gypenoside XVII (Fig. 4b). To achieve a suitable conversion yield and product concentration, we selected 4 g gypenoside XVII l−1 as the substrate concentration.
Effects of enzyme and substrate concentrations on the production of F2 from gypenoside XVII by β-glucosidase from T. thermophiles. a Effect of enzyme concentration. The reactions were performed in 50 mM citrate/phosphate buffer (pH 6.5) containing 2 g gypenoside XVII l−1 90 °C for 20 min. b Effect of substrate concentration. Ginsenoside F2 production (filled circle) and conversion yield (open square). The reactions were performed in 50 mM citrate/phosphate buffer (pH 6.5) containing 3 g enzyme l−1 at 90 °C for 20 min. Data represent the means of three experiments, and error bars represent the standard deviation
The optimal reaction conditions for the production of F2 from g gypenoside XVII were pH 6.5, 90 °C, 3 g enzyme l−1, and 4 g gypenoside XVII l−1. Under the optimized Fig. 5 conditions, the enzyme produced 3.3 g F2 l−1 after 50 min, with a molar yield of 100 % and a productivity of 4 g l−1 h−1 (Fig. 5). The results suggested that T. thermophilus β-glucosidase is an effective producer of ginsenoside F2.
Production of F2 (filled circle) from gypenoside XVII (open circle) by β-glucosidase from T. thermophilus. The reactions were performed at 90 °C in 50 mM phosphate/citrate buffer (pH 6.5) containing 4 g l−1 gypenoside XVII and 3 g enzyme l−1. Data represent the means of three separate experiments and error bars represent the standard deviation
In summary, β-glucosidase from T. thermophilus hydrolyzed only the outer glucose at the C-20 position in ginsenosides. Because of the novel narrow substrate specificity, the enzyme completely converted gypenoside XVII to ginsenoside F2 without further hydrolysis. Therefore, β-glucosidase from T. thermophillus is an effective enzyme for the production of ginsenoside F2 from gypenoside XVII as a substrate.
References
Bae EA, Choo MK, Park EK, Park SY, Shin HY, Kim DH (2002) Metabolism of ginsenoside Rc by human intestinal bacteria and its related antiallergic activity. Biol Pharm Bull 25:743–747
Cheng LQ, Kim MK, Lee JW, Lee YJ, Yang DC (2006) Conversion of major ginsenoside Rb1 to ginsenoside F2 by Caulobacter leidyia. Biotechnol Lett 28:1121–1127
Cheng LQ, Na JR, Kim MK, Bang MH, Yang DC (2007) Microbial conversion of ginsenoside Rb1 to minor ginsenoside F2 and gypenoside XVII by Intrasporangium sp. GS603 isolated from soil. J Microbiol Biotechnol 17:1937–1943
Cho WC, Chung WS, Lee SK, Leung AW, Cheng CH, Yue KK (2006) Ginsenoside Re of Panax ginseng possesses significant antioxidant and antihyperlipidemic efficacies in streptozotocin-induced diabetic rats. Eur J Pharmacol 550:173–179
Hong H, Cui CH, Kim JK, Jin FX, Kim SC, Im WT (2012) Enzymatic biotransformation of ginsenoside Rb1 and gypenoside XVII into ginsenosides Rd and F2 by recombinant β-glucosidase from Flavobacterium johnsoniae. J Ginseng Res 36:418–424
Huang C, Wang G, Li H, Xie H, Sun J, Lv H, Lv T (2006) Sensitive and selective liquid chromatography–electrospray ionisation–mass spectrometry analysis of astragaloside-IV in rat plasma. J Pharm Biomed Anal 40:788–793
Lee SY, Kim GT, Roh SH, Song JS, Kim HJ, Hong SS, Kwon SW, Park JH (2009) Proteome changes related to the anti-cancer activity of HT29 cells by the treatment of ginsenoside Rd. Pharmazie 64:242–247
Lee GW, Kim KR, Oh DK (2012) Production of rare ginsenosides (compound Mc, compound Y and aglycon protopanaxadiol) by β-glucosidase from Dictyoglomus turgidum that hydrolyzes β-linked, but not α-linked, sugars in ginsenosides. Biotechnol Lett 34:1679–1686
Andreea Neculai M, Ivanov D, Bernards MA (2009) Partial purification and characterization of three ginsenoside-metabolizing β-glucosidases from Pythium irregulare. Phytochemistry 70:1948–1957
Quan LH, Piao JY, Min JW, Kim HB, Kim SR, Yang DU, Yang DC (2011) Biotransformation of ginsenoside Rb1 to prosapogenins, gypenoside XVII, ginsenoside Rd, ginsenoside F2, and compound K by Leuconostoc mesenteroides DC102. J Ginseng Res 35:344–351
Quan LH, Kim YJ, Li GH, Choi KT, Yang DC (2013) Microbial transformation of ginsenoside Rb1 to compound K by Lactobacillus paralimentarius. World J Microbiol Biotechnol 29:1001–1007
Shin KC, Oh DK (2013) Characterization of a novel recombinant β-glucosidase from Sphingopyxis alaskensis that specifically hydrolyzes the outer glucose at the C-3 position in protopanaxadiol-type ginsenosides. J Biotechnol. doi:10.1016/j.jbiotec.2013.11.026
Shin JY, Lee JM, Shin HS, Park SY, Yang JE, Cho SK, Yi TH (2012) Anti-cancer effect of ginsenoside F2 against Glioblastoma Multiforme in xenograft model in SD rats. J Ginseng Res 36:86–92
Wang L, Liu QM, Sung BH, An DS, Lee HG, Kim SG, Kim SC, Lee ST, Im WT (2011a) Bioconversion of ginsenosides Rb1, Rb2, Rc and Rd by novel β-glucosidase hydrolyzing outer 3-O glycoside from Sphingomonas sp. 2F2: cloning, expression, and enzyme characterization. J Biotechnol 156:125–133
Wang L, Zhang Y, Chen J, Li S, Wang Y, Hu L, Wu Y (2011b) Immunosuppressive effects of ginsenoside-Rd on skin allograft rejection in rats. J Surg Res 176:267–274
Xu QF, Fang XL, Chen DF (2003) Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmacol 84:187–192
Yoshikawa M, Morikawa T, Kashima Y, Ninomiya K, Matsuda H (2003) Structures of new dammarane-type triterpene saponins from the flower buds of Panax notoginseng and hepatoprotective effects of principal ginseng saponins. J Nat Prod 66:922–927
Acknowledgments
This work was supported by the Basic Research Lab Program (No. 2010-0019306), the National Research Foundation, the Ministry of Education, Science and Technology, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shin, KC., Seo, MJ., Oh, HJ. et al. Highly selective hydrolysis for the outer glucose at the C-20 position in ginsenosides by β-glucosidase from Thermus thermophilus and its application to the production of ginsenoside F2 from gypenoside XVII. Biotechnol Lett 36, 1287–1293 (2014). https://doi.org/10.1007/s10529-014-1472-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1472-y