Abstract
Malic enzyme (EC 1.1.1.40) converts l-malate to pyruvate and CO2 providing NADPH for metabolism especially for lipid biosynthesis in oleaginous microorganisms. However, its role in the oleaginous yeast, Yarrowia lipolytica, is unclear. We have cloned the malic enzyme gene (YALI0E18634g) from Y. lipolytica into pET28a, expressed it in Escherichia coli and purified the recombinant protein (YlME). YlME used NAD+ as the primary cofactor. Km values for NAD+ and NADP+ were 0.63 and 3.9 mM, respectively. Citrate, isocitrate and α-ketoglutaric acid (>5 mM) were inhibitory while succinate (5–15 mM) increased NADP+- but not NAD+-dependent activity. To determine if fatty acid biosynthesis could be increased in Y. lipolytica by providing additional NADPH from an NADP+-dependent malic enzyme, the malic enzyme gene (mce2) from an oleaginous fungus, Mortierella alpina, was expressed in Y. lipolytica. No significant changes occurred in lipid content or fatty acid profiles suggesting that malic enzyme is not the main source of NADPH for lipid accumulation in Y. lipolytica.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
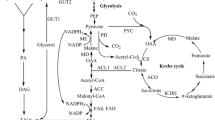
Oleaginous microorganisms, by the definition of Thorpe and Ratledge (1972), accumulate lipid to more than 20 % of their cell dry weight. The biochemistry of lipid accumulation has been studied in relatively few oleaginous yeasts and filamentous fungi: the two key events are the provision of acetyl-CoA in the cytosol to serve as the substrate for fatty acid synthase (FAS) and of NADPH for the two reductive reactions of FAS. NADPH can be produced by only a few enzymes of which malic enzyme and glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of the pentose phosphate pathway are prime candidates. Malic enzyme plays a key role in lipid accumulation in oleaginous filamentous fungi, Mucor circinelloides and Mortierella alpina (Wynn et al. 2001) and probably in several oleaginous yeasts as well (Evans and Ratledge 1985).
Malic enzyme catalyzes the oxidative decarboxylation of malate to pyruvate and CO2 in the presence of Mg2+ or Mn2+ with the concomitant reduction of NAD+ or NADP+ (Voegele et al. 1999). In some oleaginous microorganisms, malic enzyme is the major enzyme that can provide NADPH for fatty acid biosynthesis and therefore is vital for lipid accumulation (Ratledge and Wynn 2002). Over-expression of malic enzyme in the oleaginous fungus, Mcr. circinelloides, led to a 2.5-fold increase in lipid accumulation (Zhang et al. 2007). Furthermore, the lipid content in the cell decreased from 24 to 2 %, when Mcr. circinelloides was cultured in the presence of sesamol which is a specific inhibitor of malic enzyme activity (Wynn et al. 1997).
The role of malic enzyme in oleaginous yeasts is less clear than in filamentous fungi. NADP+-dependent malic enzyme activities have been detected in several oleaginous yeasts (Evans and Ratledge 1984; Li et al. 2013). Over-expression of NADP+-dependent malic enzyme from Mcr. circinelloides in oleaginous yeast Rhodotorula glutinis increased the lipid content from 19 % of the biomass to 39 % (Li et al. 2013). In Lipomyces starkeyi, however, malic enzyme is NAD+-dependent and thus is unlikely to be involved in lipid accumulation (Tang et al. 2010). The oleaginous yeast, Y. lipolytica, can accumulate lipid up to about 36 % of its biomass (Ageitos et al. 2011) and has been considered as a model organism both for laboratory study and industrial applications due to its available genome sequence and the existence of genetic tools for gene manipulation (Beopoulos et al. 2009; Blazeck et al. 2011; Wang et al. 2011). So far, extensive studies had been carried out to decipher the biochemistry and molecular mechanism of lipid accumulation in this organism (Athenstaedt 2011; Zhang et al. 2012a; Beopoulos et al. 2008). However, it is not clear whether NADPH for lipid biosynthesis arises from malic enzyme or from other sources. Genomic data (Dujon et al. 2004) shows that there is only one malic enzyme gene (YALI0E18634 g) in Y. lipolytica and which may be located in the mitochondria. Over-expression of this mitochondrial malic enzyme (YALI0E18634p) did not alter the lipid content in Y. lipolytica (Beopoulos et al. 2009, 2011).
In this study, we have cloned the malic enzyme gene from Y. lipolytica, expressed it in E. coli BL21(DE3), purified the protein and determined its biochemical characteristics. Furthermore, an NADP+-dependent malic enzyme gene from Mort. alpina, that increased the lipid content in Mcr. circinelloides when cloned into this fungus (Zhang et al. 2007), was over-expressed in Y. lipolytica Polh, to determine if the NADPH being produced by malic enzyme within the cytosol would lead to the increase of lipid accumulation in this commercially-important yeast. This strain of Y. lipolytica is now generally used for biochemical studies involving genetic manipulation (Beopoulos et al. 2008; Tai and Stephanopoulos 2013).
Materials and methods
Microorganisms and culture conditions
E. coli strains DH5α (for plasmid construction) and BL21 (for recombinant protein expression) were grown in LB medium with 100 μg ampicillin/ml or 30 μg kanamycin/ml. Mort. alpina (ATCC 32222) was grown in K & R medium with diammonium tartrate as N source and glucose as C source (Kendrick and Ratledge 1992). YPD and YNBD media (Barth and Gaillardin 1996) were prepared for Y. lipolytica (DSMZ 1345) and the auxotrophic strain Y. lipolytica Polh (kindly provided by Prof. Catherine Madzak, Institute National de la Recherche Agronomique/AgroParisTech, France) as described previously. Modified K & R medium (nitrogen-limited medium) which contained 100 g glycerol/l and 2 g ammonium tartrate/l was used to cultivate Y. lipolytica for lipid accumulation.
Construction of plasmids and purification of recombinant malic enzyme
The malic enzyme gene Ylme from Y. lipolytica and mce2 from Mort. alpina were amplified from their genomic DNA and complementary DNA respectively, with primers Ylme-F (CGC GGA TCC ATG TTA CGA CTA CGAACC AT)/Ylme-R (CGC GGA TCC CTA GTC GTA ATC CCG CAC AT) and mce2-F (CGC CAC GTG ATG ACT GTC AGC GAAAAC ACC)/mce2-R (CGC GGA TCC TTA GAG GTG AGG GGC AAA GG) respectively using standard methods. DNA fragments were first cloned into pMD19-T vector and then sub-cloned into the expression vector. The constructed plasmids were designated as pET28a-YLme and PINA1312-mce2, respectively. E. coli BL21 transformed with pET28a-YLme was cultured in 200 ml LB medium until the OD600 reached approx. 0.6. IPTG at 0.4 mM was then added to induce protein expression. Cell pellets were collected by centrifugation, re-suspended in the lysis buffer [100 mM KH2PO4, 1 mM DTT, 1 mM benzamidine hydrochloride, 20 % (v/v) glycerol, pH 7.5] and purified by affinity chromatography on a Ni–NTA column. The protein concentration was determined by Bradford’s method with BSA as a standard. SDS-PAGE was performed using 12 % (v/v) acrylamide gel. The whole purification process was carried out at 4 °C. The purified protein was stored at −80 °C in 40 % (v/v) glycerol.
Protein expression, isolation and analysis in Y. lipolytica
Yeast transformation was carried out by the lithium acetate method (Barth and Gaillardin 1996) and Y. lipolytica mutants were selected by plating on YNBD. The mutants were then cultivated in nitrogen-limited K & R medium with shaking (200 rpm) for 84 h at 28 °C for lipid accumulation. Cell pellets were resuspended in breakage buffer [50 mM sodium phosphate pH 7.4, 1 mM EDTA, 5 % (v/v) glycerol, 1 mM PMSF, pH 7.5] with the addition of an equal volume of acid-washed glass beads (0.5 mm). The disrupted cell suspensions were centrifuged at 12,000×g for 30 min at 4°C and the supernatant was used for malic enzyme analysis.
Biomass and cell lipid analysis
Biomass was harvested by centrifugation, washed twice with distilled water and dried at 110 °C to a constant weight. Lipid was extracted from lyophilized cells with chloroform/methanol (2:1, v/v) and fatty acid profiles were analyzed as their methyl esters as described by Zhang et al. (2012b).
Malic enzyme activity assays
Malic enzyme activity was assessed according to the method of Hsu & Lardy (1969) with minor modifications. The reaction mixture contained 80 mM KH2PO4/KOH pH 7.5, 3 mM MgCl2, 0.6 mM NAD(P)+, 30 mM l-malate, 8 μg protein for NAD+ assays or 80 μg for NADP+ assays in 1 ml. The formation of NAD(P)H was monitored continuously at 340 nm at 30 °C. One unit of enzyme activity was defined as the amount of enzyme required to produce of 1 nmol NAD(P)H per min. Each enzyme assay was performed in triplicate.
Results
Construction of recombinant plasmid and expression of YlME
Malic enzyme gene (YALI0E18634 g) from Y. lipolytica was amplified, cloned into the pET28a vector and transformed to E. coli BL21 (DE3) for protein expression. Optimal expression of the recombinant protein was achieved at 20 °C with 0.2 mM IPTG. The recombinant protein was purified by affinity chromatography on a Ni–NTA column, dialyzed overnight at 4 °C and analyzed by SDS-PAGE (Fig. 1) which indicated that the recombinant protein was purified to near-homogeneity.
Expression and purification of YlME in E. coli. M marker, Lane 1 crude extract of E. coli BL21(DE3)/pET28a-YLme before induction with IPTG, lane 2 crude extract of E. coli BL21(DE3)/pET28a-YLme after induction with IPTG, lane 3 the purified YlME through a Ni–NTA column (see “Materials and methods”)
Properties of malic enzyme in Y. lipolytica
Optimal malic enzyme activity was at pH 8 with either NAD+ or NADP+ as cofactor (Fig. 2a). The highest activity of YlME was at 30 °C with no measurable activity above 60 °C (Fig 2b). YlME activity decreased much faster when using NAD+ rather than NADP+ above 30 °C. The Km values for NAD+ and NADP+ were 0.63 and 3.9 mM, respectively. The Vmax values for NAD+ and NADP+ were 1.1 × 104 and 476 U/mg, respectively (Table 1).
The recombinant enzyme was dependent on Mg2+ for activity with maximum activity at 6 mM, (Fig. 2c). For NAD+-dependent malic enzyme activity, no activity was detected when Mg2+ was omitted from the reaction mixture, while for NADP+-dependent malic enzyme activity, only a low activity was detected without Mg2+, however when 0.1 mM EDTA was added to the reaction mixture, YlME activity was not detected, suggesting that metal ion (Mg2+) is absolutely required for both NAD+- and NADP+-dependent activity of YlME.
Effect of intermediate metabolites on YlME activity
To understand the role of malic enzyme in the regulation of metabolic pathways, the effect of some metabolites on YlME activity was investigated (see Fig. 3). Both citrate and isocitrate had similar effects on YlME activity (Fig. 3a, b). Below 4 mM, citrate and isocitrate had little effect on the NAD+-dependent activity but caused 40 % inhibition of NADP+-dependent activity of YlME. However, above 5 mM, both NAD+ and NADP+-dependent malic enzyme activity were strongly inhibited in a dose-dependent manner, especially NADP+-dependent activity (Fig. 3a, b). At a combined concentration of 10 mM, citrate and isocitrate also affected YlME activity: highest inhibition was with 7.5 mM citrate and 2.5 mM isocitrate when NAD+ used as a cofactor, while lowest inhibitory effect was with 5 mM citrate and 5 mM isocitrate when NADP+ used as a cofactor (Fig. 4). α-Ketoglutaric acid inhibited the NAD+ and NADP+-dependent YlME activity in a dose-dependent manner (Fig. 3c). Succinic acid at 5–15 mM significantly induced the NADP+ but not NAD+-dependent activity of YlME, while at 15–25 mM it inhibited the activity (Fig. 3d). Oxaloacetic acid at 4 mM or below inhibited NAD+- but not NADP+-dependent activity; however, above 4 mM it inhibited both NAD+ and NADP+-dependent activity (Fig. 3e). Oxalic acid inhibited the activity by 50 % at 1 mM, and 80 % at more than 6 mM, of both NAD+ and NADP+-dependent malic enzyme activity (Fig. 3f).
Relative activity of YlME detected at the addition of different concentration of intermediate metabolites using NAD+ (circular) or NADP+ (square) as a cofactor, at a constant malate concentration of 30 mM. A citrate, B isocitrate, C α-ketoglutaric acid, D succinic acid, E oxalacetic acid, F oxalic acid
Over-expression of malic enzyme in Y. lipolytica
Homologous over-expression of the NAD+-dependent malic enzyme (YALI0E18634p) does not alter lipid accumulation in Y. lipolytica (Beopoulos et al. 2009, 2011). To clarify whether malic enzyme could be involved in lipid accumulation in Y. lipolytica, an “oleaginous malic enzyme” gene (mce2) that plays a key role in lipid accumulation in Mcr. circinelloides and Mort. alpina and codes for an NADP+-dependent malic enzyme in the cytosol, was cloned from the latter source into vector PINA1312 and expressed in Y. lipolytica. The NADP+-dependent malic enzyme activity was much higher in Y. lipolytica/PINA1312-mce2 (250–350 nmol/mg min, the mutation strain) than Y. lipolytica/PINA1312 (below 1 nmol/mg.min, the control strain with empty vector) (Table 2). Although increased NADP+-dependent malic enzyme activity was recorded at different culture times of the recombinant grown in nitrogen-limited (high C/N ratio) medium, no significant change in lipid accumulation or fatty acid profiles was observed (Tables 2, 3).
Discussion
Y. lipolytica has considerable potential to be used as a cell factory for oil production (Beopoulos et al. 2010). It can grow very efficiently on several carbon sources (alkanes, fatty acid, ethanol, acetate, glucose, fructose or glycerol) and accumulate more than 40 % lipid of their biomass. It is now used commercially to produce the polyunsaturated fatty acid, eicosapentaenoic acid (EPA), using a genetically-modified strain (Xue et al. 2013). However the mechanism of lipid accumulation in this yeast is still unclear. Although malic enzyme plays a key role in lipid accumulation in oleaginous filamentous fungi, Mcr. circinelloides and Mort. alpina, its role in Y. lipolytica is still not clear.
In this study, we found that malic enzyme from Y. lipolytica can use both NAD+ and NADP+ as cofactor, but clearly prefers NAD+ to NADP+ with the NADP+-dependent activity being only about 1 % of NAD+-dependent activity (This was not due to a possible contamination of NAD+ in the commercial NADP+ preparation as purity analysis showed that it contained no significant amount of NAD+, data not shown). Also, the Km value for NAD+ was much lower than that for NADP+. This was similar to another malic enzyme from oleaginous yeast, Lipomyces starkeyi, (Tang et al. 2010) and was interpreted that malic enzyme was unlikely to be involved in lipid accumulation in this yeast.
From BLAST analysis, the fingerprint region of NAD(P)+-binding site of malic enzyme is characterized by a glycine-rich sequence (Fig. 4, Box1). However other sites may affect the binding specificity of NAD(P)+ in malic enzymes (Pon et al. 2011). All malic enzymes have a conserved domain (X1-Asp-Ser-X2-Leu, Box2), and when replaced with a valine or lysine residue, they then have NADP+-dependent activity. Malic enzyme in Y. lipolytica shares common NAD(P)+-binding sites with other malic enzymes but the second conserved domain (Fig. 5, Box2) was different from NADP+-dependent malic enzymes which led us to conclude that NAD+ is a more suitable substrate for the binding site. Our experimental results showing that malic enzyme in Y. lipolytica prefers NAD+ over NADP+, concurs with the alignment results.
BLAST Analyses (SwissProt database) of protein sequence alignments of various malic enzymes. Malic enzymes were from human mitochondria (HmNADP-ME, HmNAD(P)-ME), human cytoplasma (HcNADP-ME, HcNAD-ME), Zea mays (ZmNADP-ME), Mucor circinelloides (MtNADP-ME), Mesembryanthemum crystallinum (McNADP-ME), Mortierella alpina (MaNADP-ME), Chlamydomonas reinhardtii (CrNADP-ME), Y. lipolytica (YlME), Lipomyces starkeyi (LsNAD(P)-ME), E. coli (EcNAD-ME), Mus musculus (MmNAD-ME). Box 1 NAD(P)+ binding domain, Box 2 domain associated with NAD+ versus NADP+ preference
Malic enzyme (YALI0E18634p) in Y. lipolytica is located in the mitochondria (Beopoulos et al. 2009, 2011) where it catalyzes the oxidative decarboxylation of malate to pyruvate which then provides acetyl-CoA into Kreb’s cycle and simultaneously generates NADH for coupling into oxidative phosphorylation and energy production. The second role that has been suggested (Pon et al. 2011) may be to provide NADPH for the detoxification of reactive oxygen radicals generated in mitochondria during respiration (Pon et al. 2011). As we show here, most of the intermediates of the Kreb’s cycle, with the exception of succinic acid, inhibit the activity of malic enzyme. Structural analogues of L-malate (especially organic acids with a 2-hydroxyl or 2-keto group) behaved as the most potent inhibitors of malic enzyme activity (e.g., citrate, isocitrate, oxaloacetic acid and α-ketoglutaric) (Su et al. 2009).
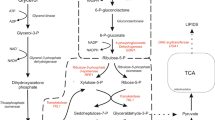
Homologous over-expression of malic enzyme (YALI0E18634p) did not affect lipid accumulation in Y. lipolytica presumably because the enzyme has low affinity for NADP+ to provide NADPH, and also because it is located in the mitochondria (Beopoulos et al. 2009, 2011). Physiologically, NADP+-dependent malic enzyme activity was too low to be detected, although NAD-dependent malic enzyme activity was high, in the transformed Y. lipolytica. Furthermore the results of transcriptomic analysis indicated that the expression of malic enzyme (YALI0E18634p) did not seem to be regulated during lipid accumulation in Y. lipolytica (Morin et al. 2011). For further confirmation, a heterologous cytosolic NADP+-malic enzyme gene (mce2) from oleaginous fungus, Mort. alpina, was expressed in Y. lipolytica, however the lipid content and fatty acid profiles were still retained at their original levels. Over-expression of malic enzyme may provide more NADPH in the cytoplasm but this NADPH was obviously not used for lipid biosynthesis. In oleaginous yeasts, L. starkeyi and Rhodosporidium toruloides, the protein level of 6-phosphogluconate dehydrogenase was upregulated in the process of lipid accumulation (Liu et al. 2009, 2011). Results of metabolic-flux and network analysis in Y. lipolytica showed that flux of pentose phosphate pathway was very high (Blank et al. 2005). Therefore it is very likely that NADPH for lipid biosynthesis is provided mainly by pentose phosphate pathway in Y. lipolytica.
In conclusion, the recombinant enzyme derived from the malic enzyme gene (YALI0E18634 g) from Y. lipolytica was purified and its biochemical characteristics were determined. It preferred NAD+ to NADP+ as a cofactor and heterologous over-expression of an “oleaginous” malic enzyme gene in this yeast did not alter the lipid content and fatty acid profiles, suggesting that other NADPH-producing enzymes, such as those in the pentose phosphate pathway, rather than malic enzyme are involved in lipid accumulation.
References
Ageitos JM, Vallejo JA, Veiga-Crespo P, Villa TG (2011) Oily yeasts as oleaginous cell factories. Appl Microbiol Biotechnol 90:1219–1225
Athenstaedt K (2011) YALI0E32769g (DGA1) and YALI0E16797g (LRO1) encode major triacylglycerol synthases of the oleaginous yeast Yarrowia lipolytica. Biochim Biophys Acta 1811:587–596
Barth G, Gaillardin C (1996) Non-conventional yeasts in biotechnology. Springer, Berlin
Beopoulos A, Mrozova Z, Thevenieau F, Le Dall MT, Hapala I, Papanikolaou S, Chardot T, Nicaud JM (2008) Control of lipid accumulation in the yeast Yarrowia lipolytica. Appl Environ Microbiol 74:7779–7789
Beopoulos A, Chardot T, Nicaud JM (2009) Yarrowia lipolytica: a model and a tool to understand the mechanisms implicated in lipid accumulation. Biochimie 91:692–696
Beopoulos A, Desfougéres T, Sabirova J, Nicaud JM (2010) Yarrowia lipolytica as a cell factory for oleochemical biotechnology. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin, pp 3003–3010
Beopoulos A, Nicaud JM, Gaillardin C (2011) An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl Microbiol Biotechnol 90:1193–1206
Blank LM, Lehmbeck F, Sauer U (2005) Metabolic-flux and network analysis in fourteen hemiascomycetous yeasts. FEMS Yeast Res 5:545–558
Blazeck J, Liu L, Redden H, Alper H (2011) Tuning gene expression in Yarrowia lipolytica by a hybrid promoter approach. Appl Environ Microbiol 77:7905–7914
Dujon B, Sherman D, Fischer G et al (2004) Genome evolution in yeasts. Nature 430:35–44
Evans CT, Ratledge C (1984) Effect of nitrogen source on lipid accumulation in oleaginous yeasts. Microbiology 30:1693–1704
Evans CT, Ratledge C (1985) The physiological significance of citric acid in the control of metabolism in lipid-accumulating yeasts. Biotechnol Genet Eng Rev 3:349–375
Hsu RY, Lardy HA (1969) Malic enzyme. Methods Enzymol 13:230–235
Kendrick A, Ratledge C (1992) Lipid formation in the oleaginous mould Entomophthora exitalis grown in continuous culture: effects of growth rate, temperature and dissolved oxygen tension on polyunsaturated fatty acids. Appl Microbiol Biotechnol 3:18–22
Li Z, Sun H, Mo X, Li X, Xu B, Tian P (2013) Overexpression of malic enzyme (ME) of M. circinelloides improved lipid accumulation in engineered Rhodotorula glutinis. Appl Microbiol Biotechnol 97:4927–4936
Liu H, Zhao X, Wang F, Li Y, Jiang X, Ye M, Zhao ZK, Zou H (2009) Comparative proteomic analysis of Rhodosporidium toruloides during lipid accumulation. Yeast 26:553–566
Liu H, Zhao X, Jiang X, Wang F, Zhang S, Ye M, Zhao ZK, Zou H (2011) The proteome analysis of oleaginous yeast Lipomyces starkeyi. FEMS Yeast Res 11:42–51
Morin N, Cescut J, Beopoulos A, Lelandais G, Le Berre V, Uribelarrea JL, Molina-Jouve C, Nicaud JM (2011) Transcriptomic analyses during the transition from biomass production to lipid accumulation in the oleaginous yeast Yarrowia lipolytica. PLoS ONE 6:e27966
Papanikolaou S, Chatzifragkou A, Fakas S, Panayotou MG, Komaitis M, Nicaud JM, Aggelis G (2009) Biosynthesis of lipids and organic acids by Yarrowia lipolytica strains cultivated on glucose. Eur J Lipid Sci Technol 111:1221–1232
Pon J, Napoli E, Luckhart S, Giulivi C (2011) Mitochondrial NAD+-dependent malic enzyme from Anopheles stephensi: a possible novel target for malaria mosquito control. Malar J 26(10):318. doi:10.1186/1475-2875-10-318
Ratledge C, Wynn JP (2002) The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv Appl Microbiol 51:1–51
Su KL, Chang KY, Hung HC (2009) Effects of structural analogues of the substrate and allosteric regulator of the human mitochondrial NAD(P)+-dependent malic enzyme. Bioorg Med Chem 17:5414–5419
Tai M, Stephanopoulos G (2013) Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production. Metab Eng 15:1–9. doi:10.1016/j.ymben.2012.08.007
Tang W, Zhang S, Tan H, Zhao ZK (2010) Molecular cloning and characterization of a malic enzyme gene from the oleaginous yeast Lipomyces starkeyi. Mol Biotechnol 45:121–128
Thorpe RF, Ratledge C (1972) Fatty acid distribution in triglycerides of yeasts grown on glucose or n-Alkanes. Microbiology 72:151–163
Voegele RT, Mitsch MJ, Finan TM (1999) Characterization of two members of a novel malic enzyme class. Biochim Biophys Acta 1432:275–285
Wang JH, Hung W, Tsai SH (2011) High efficiency transformation by electroporation of Yarrowia lipolytica. J Microbiol 49:469–472
Wynn JP, Kendrick A, Ratledge C (1997) Sesamol as an inhibitor of growth and lipid metabolism in M. circinelloides via its action on malic enzyme. Lipids 32:605–610
Wynn JP, Hamid AA, Li Y, Ratledge C (2001) Biochemical events leading to the diversion of carbon into storage lipids in the oleaginous fungi M. circinelloides and M. alpina. Microbiology 147:2857–2864
Xue ZX, Sharpe PL, Hong SP, Yadav1 NS et al (2013) Sustainable source of omega-3 eicosapentaenoic acid by metabolic engineering of Yarrowia lipolytica. Nature Biotech (in press). doi:10.1038/nbt.2622
Yu Q, Liu J, Wang Z, Nai J, Lü M, Zhou X, Cheng Y (2013) Characterization of the NADP-malic enzymes in the woody plant Populus trichocarpa. Mol Biol Rep 40:1385–1396
Zhang Y, Adams IP, Ratledge C (2007) Malic enzyme: the controlling activity for lipid production? Overexpression of malic enzyme in M. circinelloides leads to a 2.5-fold increase in lipid accumulation. Microbiology 153:2013–2025
Zhang B, Rong C, Chen H, Song Y, Zhang H, Chen W (2012a) De novo synthesis of trans-10, cis-12 conjugated linoleic acid in oleaginous yeast Yarrowia lipolytica. Microb Cell Fact 11:51. doi:10.1186/1475-2859-11-51
Zhang H, Damude HG, Yadav NS (2012b) Three diacylglycerol acyltransferases contribute to oil biosynthesis and normal growth in Yarrowia lipolytica. Yeast 29:25–38
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31271812,81071685,21276108), the National High Technology Research and Development Program of China (863 Program 2012AA022105C), the National Basic Research Program of China 973 Program (2012CB720802), the National Science Fund for Distinguished Young Scholars (31125021), the 111 project B07029, Starting Grant from Institut Mérieux (Strategic Mérieux Research Grants), and Institut Mérieux Fellowship 2012.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H., Zhang, L., Chen, H. et al. Regulatory properties of malic enzyme in the oleaginous yeast, Yarrowia lipolytica, and its non-involvement in lipid accumulation. Biotechnol Lett 35, 2091–2098 (2013). https://doi.org/10.1007/s10529-013-1302-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-013-1302-7