Abstract
A double-chamber bioreactor has been developed to generate a tissue-engineered osteochondral composite (TEOC). However, a TEOC generally requires two types of cells (i.e. chondrogenic and osteogenic cells). Therefore, the capacity of mesenchymal stem cells (MSCs) as a single-cell source to work within a double-chamber bioreactor and biphasic scaffolds for generating TEOC was investigated. Compared with static culture, the double-chamber bioreactor not only can promote faster cellular proliferation, indicated by the PicoGreen dsDNA assay, SEM and confocal imaging, but also can trigger efficient chondrogenic and osteogenic differentiation of MSCs in biphasic scaffolds simultaneously, evidenced by gene expression. Thus MSCs are promising as the ideal single-cell source and the double-chamber bioreactor is an advanced culture system to generate TEOC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Repair of cartilage defect remains a major clinical challenge due to its avascular nature and lower self-rejuvenation capacity (Hui et al. 2013). The development of regenerative medicine becomes a promising therapeutic strategy to provide substitutes for tissue-engineered cartilage (TEC) having the necessary biological features. However, implanted TEC tissue in the defect region experiences severe depression due to the lack of sufficient subchondral support (Malafaya and Reis 2009). Therefore to generate a TE osteochondral composite (TEOC) with both cartilage and underlying bone tissue component becomes a better strategy for effective healing of cartilage defects in healing cartilage defect.
Considering the different intrinsic properties of cartilage and bone tissues, it becomes difficult to generate TEOC within a single system due to the use of different culture media and differentiation conditions. To overcome this problem, a double-chamber bioreactor consisting of one chamber for culturing the chondrogenic part and another chamber for the osteogenic component was developed (Malafaya and Reis 2009). Two different types of cells, chondrogenic cells (e.g. chondrocytes) and osteogenic cells (e.g. osteoblasts), are though generally needed for the development of TEOC within the double-chamber bioreactor (Malafaya and Reis 2009; Chang et al. 2004). This requires two separate cell handling processes to isolate, culture and expand the cells before seeding to scaffolds, hence more complicated operation procedures, higher risk of contamination and greater economic cost.
With their unique capability to undergo both osteogenic and chondrogenic differentiation, mesenchymal stem cells (MSCs) exhibit a great potential as the unified cellular source for TEOC, which may considerably simplify the preparation procedures. Therefore, in this study, we have evaluated the potential use of MSCs alone to develop TEOC by seeding them into biphasic scaffolds and culturing cell-scaffold composites in a specifically designed double-chamber bioreactor for simultaneous osteogenic and chondrogenic differentiation in the different phases (Table 1).
Materials and methods
Preparation of a biphasic scaffold
The osseous phase was made from β-tricalcium phosphate (β-TCP) (Sohier et al. 2010). Briefly, an aqueous slurry of β-TCP (Bio-lu Bio Materials Company Ltd.) was mixed with polymethylmethacrylate (PMMA) powders (Dentalbiolux International, Belgium) at (w/w) 1:1. The mixture was heated to 400 °C for 30 h to burn away the porogen (PMMA) and sintered at 1,000 °C for 3 h. Finally, the porous β-TCP ceramic were machined into 6 (diam.) × 4 mm3 cylinders. The chondral phase was prepared as previously described (Bi et al. 2011). A solution of 2 % (w/v) collagen type II mixed with 2 % (w/v) chitosan at a ratio of 1:3 (v/v). The osseous phase was tightly wrapped with a polypropylene film and collagen/chitosan mixture was added on one side. Glue, made of cross-linked 1.2 % (w/v) sodium alginate and CaCl2, was used before the adding of the mixture to stick and separate the two phases. Then this film-wrapped composite was lyophilized to form biphasic cylinder of 6 (diam.) × 8 mm3. Each cylinder had a 4 mm thick osseous phase and a 4 mm thick chondral phase. The morphological characteristics were examined by a scanning electron microscope.
Preparation of a double-chamber bioreactor (see Fig. 1)
The double-chamber bioreactor consists of two tubular-shape glass chambers and each has four branch tubes. The chambers are separated by a silicone-rubber membrane, which possesses a hole in its center for holding one biphasic scaffold. Magnetic-bar stirring provides mechanical stimulation. The entire system can be sterilized and placed into an incubator.
A scheme of the design and working pattern of the double-chamber bioreactor system (a). The bioreactor consists of two tubular-shape glass chambers, each of which has three branch tubes: one for medium inflow and one for outflow and one for ventilation. The chambers are separated by a silicone-rubber membrane, which possesses a hole in its center for holding one biphasic scaffold. Magnetic-bar stirring provides mixing of the medium and mechanical stimulation. Photograph of the biphasic scaffold after cross-linking. The chondral phase (b) and the osseous phase (c)
Isolation and culture of goat MSCs
MSCs were isolated from ileums of adult Chinese goats. Nucleated cells were isolated and resuspended in DMEM (Hyclone) containing 10 (w/v) FBS and 1 % (w/v) penicillin/streptomycin. The cells were incubated in a humidified atmosphere of 95 % air and 5 % CO2 at 37 °C. Non-adherent cells were discarded after 2 days, and the adherent cells were cultured for further expansion. When cultured, cells became near-confluent and were detached by 0.25 % (w/v) trypsin and reseeded for continued passage. The medium was changed every 2 days.
Cell seeding and culture in the bioreactor
The third-passage MSCs were trypsinized and resuspended at 2 × 106 cells/ml in DMEM. The cell suspension, 0.2 ml was slowly injected into one biphasic scaffold (0.1 ml into chondral phase and 0.1 ml into osseous phase). Mild negative pressure was applied during cell seeding. Every cell-containing scaffold was held at the interface of the two phases by the silicone-rubber membrane in the bioreactor. The junction between the scaffold and the membrane was sealed with glue. The bioreactor had two independent medium-circulation systems and supplied two types of media for chondrogenic or osteogenic differentiation. The chondrogenic medium was DMEM containing 6.25 μg insulin/ml, 6.25 μg transferrin/ml, 50 μg ascorbic acid/ml, 10−8 M dexamethasone (DEX) and 10 ng TGF-β1/ml (PeproTech). The osteogenic medium was DMEM containing 10 % (v/v) FBS, 10−8 M DEX, 10−3 M β-glycerol phosphate, and 50 mg l-ascorbic acid/ml. The bioreactor provided dynamic stimulation for cell culture with magnetic-bars stirring at a speed of 20 rpm for 1 h per day (induction medium with dynamic stimulation, i.e. ID group). Cells cultured under the other three conditions, such as induction medium with no dynamic stimulation (IN), no induction medium with dynamic stimulation (ND) and no induction medium with no dynamic stimulation (NN) were controls.
Cellular proliferation and viability
Morphologies of the MSCs were examined weekly by phase contrast light microscope and SEM at weeks 2 and 4. Analysis of cell viability was performed with fluorescein diacetate staining and imaged by a confocal laser scanning microscope. The total cell number in the scaffolds at week 0 (the time of cell seeding), 1, 2, 3 and 4 was estimated by quantifying the double-stranded DNA (dsDNA) content of each scaffold using a PicoGreen dsDNA Quantification Kit (Molecular Probes). The total dsDNA was extracted through enzymatic digestion and assayed by following the manufacturer’s instructions. The proliferation of the cells in the scaffold was interpreted by changes of dsDNA quantity. The two phases were separated before dsDNA extraction and quantified separately.
RNA analysis
For quantitative real-time RT-PCR (qRT-PCR) analyses, the cell-containing scaffolds were homogenized and frozen in liquid N2. Total RNA was extracted by Trizol and the first-strand cDNA was reverse transcribed using TaqMan reverse transcription reagents (Invitrogen) from each sample after culture for 1, 2, 3 and 4 weeks. The mRNA of chondrogenic and osteogenic markers was performed by using Power SYBR-Green (Applied Biosystems). The primer details are listed in Supplmentary Table 1. Results were normalized against the house keeping gene, and relative gene expression analyzed (Livak and Schmittgen 2011).
Statistical analysis
Statistical analyses were performed using SPSS software, version 14.0. The data were presented as mean ± SD, and levels were compared by the non-parametric Mann–Whitney U test. P values < 0.05 were considered significant.
Results
Characteristics of the biphasic scaffold
The chondral phase and osseous phase were well-connected after cross-linking. The chondral phase exhibited a sponge-like pattern of regular interconnected pores (Fig. 1b) with a porosity of 85 %. The osseous phase represented a porous structure that contained well-interconnected and regular spherical pores (Fig. 1c). Compared with chondral phase, osseous phase showed a lower porosity (70 %).
Cellular proliferation
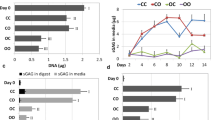
SEM showed under both dynamic and static conditions, MSCs could adhere well to the surface of scaffolds at week 2 (Fig. 2a–d) and achieved confluence at week 4 (Fig. 2e–h). However, the staining with fluorescein diacetate (FDA) at the core region at week 4 revealed that under the dynamic condition, MSCs saturated all pores throughout the whole scaffolds (Fig. 2i, j), whereas under static conditions, the pores at the core region of scaffold are devoid of cells (Fig. 2k, l). The SEM and FDA findings were further corroborated by dsDNA assay. Under the dynamic conditions (groups ID and ND), cells proliferated faster within the biphasic scaffold during the first 2 weeks and then plateaued. In contrast, the dsDNA contents from statically-cultured scaffold constructs (group IN and NN) increased very slowly from 1 to 3 week, and decreased slightly at week 4. The dsDNA contents of cellular scaffolds under dynamic culture were significantly higher than those under static culture throughout the whole study (P < 0.05). In addition, the introduction of osteogenic or chondrogenic inductive medium showed no significant influence on proliferation of MSC in the scaffolds (P > 0.05) (Fig. 2m, n).
SEM showed BMSCs (arrow) adhere fast at the surface of scaffolds under either dynamic (a: chondral phase from ID, b: osseous phase from ID) or static conditions (c: chondral phase from IN, d: osseous phase from IN) at 2 week. By the 4 week, BMSCs from ID were saturated all the pores at both the surface (e: chondral phase, f: osseous phase) and center (i: chondral phase, j: osseous phase) of the scaffolds. Complete cellular confluence only could be found at the surface of scaffolds in static culture (g: chondral phase, h: osseous phase), with large pores at the center of the scaffolds devoid of cells (red circle) being found at the end of the experiment at week 4 (k: chondral phase, l: osseous phase). The dsDNA content of ID and ND increased faster during the first 2 weeks, and plateaued since week 2. The dsDNA contents from IN and NN increased slowly from 1 to 3 week, and decreased at week 4. The dsDNA contents of dynamic culture were significantly higher than those of static culture (n = 6, P* < 0.05, P# > 0.05) (m, n)
Gene expression analysis
In terms of chondrogenic differentiation of MSCs within the chondral phase, the aggrecan and Col II transcription level in the ID group continued to rise with culture time to reach the highest level at week 4 (Fig. 3a, b), whereas, Col I showed little change during the 4 weeks (Fig. 3c, P > 0.5). For cultured cellular scaffolds in chondrogenic induction media without dynamic stimulation (IN), both the expression of aggrecan and Col II were up-regulated gradually with culture time, but their expression levels were significantly lower than those under dynamic culture (ID) from 2 to 4 week (Fig. 3a, b, P < 0.05). The up-regulation of Col I in IN group, however, was significantly higher than that of ID group throughout the last 3 weeks (Fig. 3c, P < 0.05). The up-regulation of aggrecan and Col II were negligible in the two chondrogenic medium-free groups (ND and NN) (Fig. 3a, b). Regarding the osteogenic differentiation of MSCs in osteogenic phase of scaffolds, the osteogenic induction led to a similar level of gene expression in both dynamic culture group (ID) and static culture group (IN). In particular, the expression level of three key osteogenic genes, Cbfa1, Col I and osteocalcin elevated and peaked either at week 2 (Cbfa1 and Osteocalcin) or week 3 (Col I) (Fig. 3d–f). The results of immunohistochemistry study were in line with the changes of qRT-PCR (Supplementary Fig. 1).
The aggrecan and Col II transcription level in the chondral phase from ID continued to rise with culture time to reach the highest level at the 4 week (a, b), whereas, Col I expression levels showed little change during the 4 weeks (c). In the chondral phase from IN, both the expression of aggrecan and Col II genes were up-regulated gradually with culture time, but their expression levels were significantly lower than those from ID from 2 to 4 weeks (a, b). On the contrary, the up-regulation of Col I gene in IN group were significantly higher than that of the ID group throughout the last 3 weeks (c). Regarding the osteogenic differentiation of BMSCs in osteogenic phase, the osteogenic induction led to a similar level of gene expression in both group ID and IN (d–f). (P* < 0.05, n = 6)
Discussion
The choice of scaffolds is critical for the success of regenerative medicine approaches (Abarrategi et al. 2010). In order to enhance the in situ integration of the chondral phase (chitosan/collagen scaffold) with the host cartilage tissues, we developed a biphasic osteochondral composite by combining the chondral phase with a bone-ECM mimicking scaffold, i.e. osseous phase (β-TCP).
Selection of suitable cell to promote efficient tissue regeneration is another critical consideration in regenerative medicine. Articular chondrocytes could be used immediately after isolation from native cartilage tissue without in vitro expansion and achieved certain success in small defect treatment (Appelman et al. 2011). However, in order to obtain clinically significant number of cells for large defect treatment, ex vivo expansion of articular chondrocytes are usually required, which is frequently restricted by the issue of cell de-differentiation (Appelman et al. 2011). Moreover, due to the lack of sufficient osteogenic capacity of chondrocytes, additional cell sources for osseous phase were required, making their application more complicated with additional cell harvesting, processing and culturing procedures. MSCs can be readily isolated from bone marrow and expanded through multiple passages. Additionally, they are believed to be non-immunogenic transplantation paradigms (Gala et al. 2011; Rodrigues et al. 2011) favoring their clinical application. MSCs possess higher chondrogenic and osteogenic potentials as compared to other stem cells (Niemeyer et al. 2010) making them the unique cellular candidate for generating the TEOC. In this study, MSCs can be easily and efficiently induced to differentiate into both chondrocytes and osteoblasts under appropriate induction culture system.
A bioreactor can apply favorable mechanical stimulation to facilitate chondrogenic or osteogenic differentiation of MSCs (Niemeyer et al. 2010). In our study, we found that the up-regulation of the key genes related to chondrogenic differentiation were negligible in the induction medium-free groups, which meant that dynamic stimulation alone may not be sufficient to make significant influence on the in vitro chondrogenic differentiation of MSCs. Once combined with induction media, however, the dynamic culture in the double-chamber bioreactor stimulated a much more robust chondrogenic differentiation of MSCs cultured in the scaffolds than static culture, evidenced by the gene expression.
This finding is in line with other reports showing that mechanical loading could promote chondrogenesis of MSCs through responsive gene regulating pathway (Li et al. 2012). Further studies on mechanotransduction pathways revealed that the activation of Smad signaling pathway may play a significant role in the up-regulation of chondrogenic genes and the chondrogenic differentiation of MSCs under the dynamic stimulation (Li et al. 2012). In our study, the combination of dual inductive factors (media with TGF-β1 and dynamic stimulation) has achieved the most robust chondrogenic differentiation in MSCs mediated scaffold compared to the single inductive factors. Within this bioreactor, limited diffusion of the media may occur inside the scaffold, which may facilitate microinteractions between different cells at the interface; however, little exchange takes place between the two chambers. In addition, as the cells secrete increasing amounts of matrix, diffusion will progressively decrease.
In summary: MSCs can thus be used as a single source of cells to generate TEOC with the combinational use of a double-chamber bioreactor and a biphasic scaffold. The double-chamber bioreactor not only promotes cellular proliferation but also triggers chondrogenic and osteogenic differentiation of MSCs in biphasic scaffolds simultaneously. The combination of double-chamber bioreactor and biphasic scaffolds facilities the application of MSCs as the single-cell source and also presents a prologue for the development of a new strategy for articular cartilage defect treatment. In order to translate this technology into the clinic, further, more detailed studies related to human MSCs are needed.
References
Abarrategi A, Lópiz-Morales Y, Ramos V, Civantos A, López-Durán L, Marco F, López-Lacomba JL (2010) Chitosan scaffolds for osteochondral tissue regeneration. J Biomed Mater Res A 95:1132–1141
Appelman TP, Mizrahi J, Elisseeff JH, Seliktar D (2011) The influence of biological motifs and dynamic mechanical stimulation in hydrogel scaffold systems on the phenotype of chondrocytes. Biomaterials 32:1508–1516
Bi L, Cao Z, Hu Y, Song Y, Yu L, Yang B, Mu J, Huang Z, Han Y (2011) Effects of different cross-linking conditions on the properties of genipin-cross-linked chitosan/collagen scaffolds for cartilage tissue engineering. J Mater Sci Mater Med 22:51–62
Chang CH, Lin FH, Lin CC, Chou CH, Liu HC (2004) Cartilage tissue engineering on the surface of a novel gelatin-calcium-phosphate biphasic scaffold in a double-chamber bioreactor. J Biomed Mater Res B Appl Biomater 71:313–321
Gala K, Burdzińska A, Idziak M, Makula J, Pączek L (2011) Characterization of bone-marrow-derived rat mesenchymal stem cells depending on donor age. Cell Biol Int 35:1055–1062
Hui JH, Ren X, Afizah MH, Chian KS, Mikos AG (2013) Oligo[poly(ethylene glycol)fumarate] hydrogel enhances osteochondral repair in porcine femoral condyle defects. Clin Orthop Relat Res 471:1174–1185
Li J, Wang J, Zou Y, Zhang Y, Long D, Lei L, Tan L, Ye R, Wang X, Zhao Z (2012) The influence of delayed compressive stress on TGF-β1-induced chondrogenic differentiation of rat BMSCs through Smad-dependent and Smad-independent pathways. Biomaterials 33:8395–8405
Livak KJ, Schmittgen TD (2011) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Malafaya PB, Reis RL (2009) Bilayered chitosan-based scaffolds for osteochondral tissue engineering: influence of hydroxyapatite on in vitro cytotoxicity and dynamic bioactivity studies in a specific double-chamber bioreactor. Acta Biomater 5:644–660
Niemeyer P, Fechner K, Milz S, Richter W, Suedkamp NP, Mehlhorn AT, Pearce S, Kasten P (2010) Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials 31:3572–3579
Rodrigues MT, Gomes ME, Reis RL (2011) Current strategies for osteochondral regeneration: from stem cells to pre-clinical approaches. Curr Opin Biotechnol 22:726–733
Sohier J, Daculsi G, Sourice S, de Groot K, Layrolle P (2010) Porous beta tricalcium phosphate scaffolds used as a BMP-2 delivery system for bone tissue engineering. J Biomed Mater Res A 92:1105–1114
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Xg., Jiang, Hk. Preparation of an osteochondral composite with mesenchymal stem cells as the single-cell source in a double-chamber bioreactor. Biotechnol Lett 35, 1645–1653 (2013). https://doi.org/10.1007/s10529-013-1248-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-013-1248-9