Abstract
Nanostructured crystalline titanium dioxide coatings deposited by cathodic arc evaporated on titanium grade five medical implant substrates were demonstrated to exhibit UV-induced photocatalytic activity that can be utilized to provide bactericidal effects against Staphylococcus epidermidis. The photocatalytic activity of the coatings was confirmed via degradation of Rhodamine B under UV illumination. A 90 % reduction of viable bacteria was achieved in a clinically suitable time of only 2 min with a UV dose of 2.4 J delivered at 365 nm. These results are encouraging for the development of antimicrobial surfaces in orthopedics and dentistry in order to prevent or treat post-surgical infections.
Purpose of work
To assess the possibility of employing photocatalysis for elimination of S. epidermidis, known to cause medical device related infections, under short enough times to be clinically useful on an implant surface produced with a technique that is suitable for mass production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Implant related infections constitute a large and growing problem within orthopedics and dentistry (Pulido et al. 2008; Costerton et al. 2005). They are difficult to treat and often lead to patient suffering and substantial societal costs (Lavernia et al. 1995). The infections arise at the implant-tissue interface, referred to as an immune-incompetent fibro-inflammatory zone (Gristina 1994). At such interfaces a local depression of the immune system occurs (Zhao et al. 2009), which makes implant surfaces extra susceptible to bacterial colonization and biofilm formation (Costerton et al. 2005). Biofilm bacteria are challenging to eliminate with systemic antibiotic therapy (Shiraishi et al. 2008), hence, the only way to combat implant related infections is, often, to remove the implant and replace it with a new one. Skin penetrating implants are especially susceptible to bacteria colonization since they are in constant contact with skin bacteria (Temple et al. 2004). Examples of such implants are external fixation wires and pins, which often become colonized by Staphylococcus aureus and S. epidermidis (Collinge et al. 1994). On skin penetrating implants, one generally finds a higher prevalence of Gram-positive bacteria (90.6 % S. epidermidis) compared to Gram-negative ones (Mahan et al. 1991).
Several strategies have been employed to develop antibacterial modifications of implant surfaces in order to prevent microbial colonization and subsequent biofilm formation (Brunski et al. 2000). These include incorporation of releasable traditional antibiotics (Brohede et al. 2009a, b; Forsgren et al. 2011a, b) and metal ions (Heidenau et al. 2005; Ewald et al. 2006).
Titanium dioxide (TiO2) is known for its biocompatibility, that becomes evident by rapid biomimetic hydroxyapatite formation upon immersion in biological fluids (Åberg et al. 2009; Piskounova et al. 2009; Mihranyan et al. 2009; Brohede et al. 2009c; Forsgren et al. 2011b; Lilja et al. 2011). It is a well-studied and clinically widely used biomaterial for bone applications with excellent chemical stability, no toxicity and good mechanical properties (Brudnik et al. 2008; Uzunova-Bujnova et al. 2008; Diebold 2003). Crystalline TiO2 is not only a bioactive, non-resorbable (Kasuga et al. 2002; Crawford et al. 2007) material promoting tissue integration (Zhu et al. 2006), it also exhibits excellent photocatalytic properties, generating photo-induced reactive oxygen species (ROS), when irradiated with UV light (Sirghi et al. 2003; Carp et al. 2004; Hossain et al. 2008; Welch et al. 2010). These properties make crystalline TiO2 very attractive as implant coatings.
Photocatalysis on crystalline TiO2 surfaces for disinfection purposes (Gamage et al. 2010; Chong et al. 2010) has been proven capable of eliminating a wide range of Gram-negative and Gram-positive bacteria. Nevertheless, there have been very few reports about its application to bio-implant-related infections (Cai et al. 1991; Choi et al. 2007, 2009; Riley et al. 2005; Welch et al. 2012), especially with focus on clinically relevant strains such as S. aureus or S. epidermidis (Gallardo-Moreno et al. 2010; Welch et al. 2010).
The photocatalytic activity (PCA) of a TiO2 surface depends on a number of factors including surface area and crystallinity. Most studies investigating the PCA of TiO2 surfaces have concluded that the anatase polymorph of TiO2 is a more effective photocatalyst compared to rutile (Miyagi et al. 2004). However, some studies indicate that a certain mixture of anatase and rutile can produce a higher PCA, and consequently an enhanced bactericidal effect, than pure anatase (Miyagi et al. 2004; Sato et al. 2006). These studies demonstrate that the use of a photocatalytic implant coating may be a viable strategy for reducing implant related infections. However, the illumination time required in these studies in order to achieve a desired antibacterial effect (in excess of an hour of UV illumination) needs to be significantly reduced for the technique to be clinically relevant. Coatings on implant surfaces have high requirements on their physical properties (Brohede et al. 2009c). Hence, cathodic arc evaporation could be an attractive alternative for the deposition procedure of such coatings as it allows for rapid deposition of TiO2 thin films in a mass production ready process with a high degree of control of the coating microstructure and excellent adhesion to the substrate (Kleiman et al. 2007).
The present study evaluates the antimicrobial effects on S. epidermidis under UV light illumination of a TiO2 coating produced by cathodic arc deposition. The particular bacterium strain is chosen because it is a common cause of medical device-associated infections (Rodriguez-Martinez and Pascual 2006), especially on skin penetrating implants (Mahan et al. 1991; Collinge et al. 1994). The aim is to evaluate if it is possible to employ photocatalysis for bacteria elimination on implant surfaces under short enough times to be clinically practical on an implant surface produced with a technique that is suitable for mass production.
Materials and methods
Coating deposition
Cylindrical substrates (Ø = 9 mm, thickness 1 mm) of commercially available titanium grade 5 (Ti–6Al–4V) were used for the arc evaporation coating process (Lilja et al. 2012). Briefly, a crystalline TiO2 coating with a pronounced anatase phase composition was deposited onto the substrates by cathodic vacuum arc evaporation during a deposition time of 30 min. For coating thickness measurements two notches of 3 mm in length were cut from both sides towards the center of the substrate prior to coating deposition.
Coating characterization
X-ray diffraction (XRD) measurements
The crystallinity of the coatings was examined using grazing incidence XRD (Siemens D5000 diffractometer). The measurements were recorded between 20 and 60° 2θ with an incidence angle of 1° using a step size of 0.1° and a scan step time of 4 s.
From the full width at half maximum (FWHM) of the anatase XRD peaks A(101), A(200) and A(004), the effective grain size of the as deposited TiO2 films was calculated by using the Scherrer equation (Guinier et al. 1994):
where L is the effective grain size and λ is the wavelength of the X-ray. Broadening caused by internal strain was not taken into account.
Scanning electron microscopy (SEM)
The TiO2 coating topography and thickness was evaluated by using a scanning electron microscope (Zeiss Supra 40). In order to be able to view the cross section of the coating and measure the coating thickness, the notched coated samples were first frozen in liquid nitrogen and then broken into two halves by applying an abrupt mechanical force.
Photocatalytic activity testing
The PCA of the as deposited TiO2 films was determined by measuring the degradation of Rhodamine B dye as a function of time as described in Lilja et al. (2012). Briefly, four coated samples were placed in a quartz cell containing 2.5 ml Rhodamine B solution (5 μM, pH ~ 6, Sigma) and irradiated with an average UV light intensity of 6.7 mW/cm2 (UV LED NCSU033B, λ = 365 nm, Nichia, Japan) at 100 Hz and 10 % duty cycle. During the 250 min measurement, the solution was stirred with a magnetic bar and the degradation of the dye was evaluated by absorption measurements at 5 min intervals using a UV-photospectrometer (UV-1800, Shimadzu).
Assuming a pseudo first order reaction rate for the Rhodamine B degradation, the degradation rate, k, which is a measure of the PCA, was obtained by fitting the dye concentration vs. time curves to the following equation (Konstantinou et al. 2001):
where C 0 is the initial dye concentration and C(t uv ) is the concentration after UV light irradiation for a given time t uv .
Antibacterial testing with UV irradiation
Gram-positive S. epidermidis (CCUG 18000A) was used as the model bacteria in the bactericidal tests. It constitutes an interesting test bed for investigating non-antibiotic bactericidal treatments since (i) it is part of the human skin flora, (ii) it has a low requirement for nutrition and forms biofilms characterized by a high degree of antibiotic resistance (Stewart et al. 2001) and (iii) it is a common cause of medical device-associated infections (Rodriguez-Martinez et al. 2006).
S. epidermidis was inoculated into 12 ml of Müller Hinton broth II (MH II) nutrition medium (Difco, Becton, Dickinson and Company, Sweden) in a 15 ml falcon tube. The tube was incubated at 37 °C overnight before the bacteria were collected by centrifugation at 1,000×g (Heraeus Primo, Thermo Scientific) and re-suspended in 300 μl of sterile distilled water. The bacteria suspension was diluted to an optical density of 1.0, corresponding to a cfu concentration of about 109/ml. Prior to antibacterial testing, the coated samples were ultrasonically cleaned for 5 min in first ethanol and then distilled water.
An 8 μl drop of bacteria suspension was spread over each sample disc before UV irradiation. The samples were irradiated with a 365 nm light source at an intensity of 20 mW/cm2 between 0 and 13 min, corresponding to UV doses between 0 and 16 J. Tests were performed in triplicates for each irradiation time. Uncoated titanium grade 5 discs were used as reference.
After UV illumination, the samples were rinsed with 0.5 ml distilled water to remove loosely adhered bacteria from the surfaces and this water was collected in individual wells of a well plate. The sample disks were then placed face down in their corresponding well with the rinsing water and the well plate was placed in an ultrasonic bath for 30 s to ensure that all the bacteria were removed from the sample surfaces. Thereafter 100 μl of bacteria suspension from each well was transferred to individual wells in a 96-well plate and diluted to 300 μl with MH II broth containing 2.5 vol% Resazurin for viability measurements. Resazurin functions as a viability indicator as it is reduced to Resorufin by the mitochondria in viable bacteria (Gonzalez et al. 2001). Since Resorufin is fluorescent, the amount of viable bacteria can be quantified by measuring the fluorescence of the medium containing the bacteria. To increase the sensitivity of the metabolic assay for low numbers of viable bacteria, the well plate was incubated for 14 h at 37 °C to allow the bacteria to multiply and produce a larger amount of Resorufin. The fluorescence measurements were performed with an Infinite M200 microplate-reader from Tecan, using an excitation wavelength of 530 nm and an emission wavelength of 590 nm. In order to correlate the fluorescent signal to the number of viable bacteria, a standard curve was made from a dilution series of the original bacteria suspension with a known cfu concentration.
Results and discussion
The XRD diffraction pattern of the as deposited TiO2 coating in Fig. 1a shows that the microstructure is dominated by the anatase phase with minor amounts of rutile phase present in the structure. It should be noted that the substrate material used in the present work is one of the most commonly used biomaterials in orthopedic and dental applications due to its favorable mechanical properties and good biocompatibility (Navarro et al. 2008). As well the anatase phase, which is the dominating phase of the deposited coatings, has in several studies been proven to be bioactive and non-toxic (Brunette et al. 2001).
The SEM image of the coating in cross section, Fig. 1b, displays that the TiO2 film has a total thickness of about 700 nm and consists of columnar grains throughout the entire coating. The SEM image of the TiO2 coating topography, Fig. 1c, shows that the coating surface consists of distinctive grains. The diameter of the grains is approximately 40–50 nm, which is consistent with the average calculated grain size of 45 nm using Eq. (1). The surface roughness of the underlying titanium substrate and the impact of the deposition process are reflected by the contoured surface topography of the coating.
Figure 2 displays the PCA of the TiO2 surface, where the Rhodamine B concentration is displayed as a function of time in a solution containing the samples under UV illumination. Compared to the Ti grade 5 reference surfaces, a clear degradation of the Rhodamine dye is observed with the coated Ti surfaces. From curve fits to Eq. (2) it is found that the TiO2 coating induce a degradation rate of k = 5.63 × 10−4/min, which is more than 20 times larger than the rate of 0.25 × 10−4/min induced by the reference surface. Since the reference surface is not expected to be photocatalytic, it is likely that the observed degradation rate in tests performed on this surface is due to the effect of the UV light itself on the Rhodamine B solution. The degradation rate measured for the TiO2 coating is comparable to previous results obtained with similar PVD coatings (Kleiman and Marquez 2007; Lilja et al. 2012). As suggested in other studies (Fox et al. 1993; Miyagi et al. 2004), the degree of PCA is likely governed by the anatase dominated phase composition of the coating in association with the uneven and coarse surface morphology that results in an increased surface area.
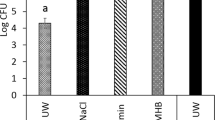
Figure 3 shows the bactericidal effects of the TiO2 coated and Ti reference surfaces under UV illumination. The coated surfaces exhibit a larger bactericidal effect than that seen with the reference surfaces when comparing the UV dose range from 1.2 J (corresponding to 1 min of illumination) up to approximately 10 J. Whereas the number of viable bacteria decreases monotonically with increasing UV dose for the coated samples, an increased bacterial viability is observed for UV doses ≤3 J for the reference samples. For higher UV doses, the number of viable bacteria is decreasing also on the reference samples. The latter can be related to the inherent bactericidal effect of UV light alone (Shiraishi et al. 2008). The greater antibacterial effect seen with the coated disks, particularly at clinically relevant UV doses less than 3 J, is attributed to the photo-induced ROS formed at the TiO2 surface, which in turn react with the S. epidermidis bacteria present on the surface, rendering them non-viable.
The observed increase in number of viable bacteria on the reference samples at low UV doses has also been reported in previous studies (Shiraishi et al. 2008) and may be ascribed to an increased, and more favorable, temperature for bacterial proliferation (Ratkowsky et al. 1981; 1983; Cooper et al. 2001).
Summary and conclusion
Cathodic arc evaporated, nano-structured anatase thin films on titanium grade 5 substrates were demonstrated to exhibit a UV-induced photocatalytic activity that can be utilized to provide a bactericidal effect against S. epidermidis. A 90 % reduction of viable bacteria was achieved in only 2 min with a UV dose of 2.4 J.
Photocatalytic elimination of bacteria has been presented in previous studies with TiO2 coatings (Panda et al. 2010; Gallardo-Moreno et al. 2010; Shiraishi et al. 2008) but the treatment time required to achieve a bactericidal effect in previous studies was on the order of one hour or more.
Post-surgical infections and infections around metallic implants associated with biofilm formation are major concerns in clinical research. Obtaining the desired antimicrobial effect in as little as 2 min should make the presented technique more clinically relevant than previously presented approaches and, thus, open up for new on-demand antimicrobial surface treatment methods for biomedical implants in order to combat infections or reduce infection risks.
References
Åberg J, Brohede U, Mihranyan A, Strømme M, Engqvist H (2009) Bisphosphonate incorporation in surgical implant coatings by fast loading and co-precipitation at low drug concentrations. J Mater Sci Mater Med 20:2053–2061
Brohede U, Forsgren J, Roos S, Mihranyan A, Engqvist H, Strømme M (2009a) Multifunctional implant coatings providing possibilities for fast antibiotics loading with subsequent slow release. J Mater Sci Mater Med 20:1859–1867
Brohede U, Forsgren J, Mihranyan A, Engqvist H, Strømme M (2009b) Fast loading, slow release—a new strategy for incorporating antibiotics to hydroxyapatite. Key Eng Mater 396–398:523–526
Brohede U, Zhao S, Lindberg F, Mihranyan A, Forsgren J, Strømme M, Engqvist H (2009c) A novel graded bioactive high adhesion implant coating. Appl Surf Sci 225:7723–7728
Brudnik A, Bucko M, Radecka M, Trenczek-Zajac A, Zakrzewska K (2008) Microstructure and optical properties of photoactive TiO2:N thin films. Vacuum 82:936–941
Brunette DM, Tengvall P, Textor M, Thomsen P (2001) Titanium in medicine. Springer, Berlin
Brunski JB, Puleo DA, Nanci A (2000) Biomaterials and biomechanics of oral and maxillofacial implants: current status and future developments. Int J Oral Max Implant 15:15–46
Cai R, Hashimoto K, Itoh K, Kubota Y, Fujishima A (1991) Photokilling of malignant cells with ultrafine TiO2 powder. Bull Chem Soc Jpn 64:1268–1273
Carp O, Huisman CL, Reller A (2004) Photoinduced reactivity of titanium dioxide. Prog Solid State Chem 32:33–177
Choi JY, Kim KH, Choy KC, Oh KT, Kim KN (2007) Photocatalytic antibacterial effect of TiO2 film formed on Ti and TiAg exposed to Lactobacillus acidophilus. J Biomed Mater Res Pt B Appl Biomater 80:353–359
Choi JY, Chung CJ, Oh KT, Choi YJ, Kim KH (2009) Photocatalytic antibacterial effect of TiO2 film on TiAg on Streptococcus mutans. Angle Orthod 79:528–532
Chong MN, Jin B, Chow CWK, Saint C (2010) Recent developments in photocatalytic water treatment technology: a review. Water Res 44(10):2997–3027
Collinge CA, Goll G, Seligson D, Easley KJ (1994) Pin tract infections: silver vs. uncoated pins. Orthopedics 17:445–448
Cooper VS, Bennett AF, Lenski RE (2001) Evolution of thermal dependence of growth rate of E. coli populations during 20,000 generations in a constant environment. Evolution 55:889–896
Costerton JW, Montanaro L, Arciola CR (2005) Biofilm in implant infections: its production and regulation. Int J Artif Organs 11:1062–1068
Crawford GA, Chawla N, Das K, Bose S, Bandyopadhyay A (2007) Microstructure and deformation behavior of bioactive TiO2 coatings. Acta Biomater 3:359–367
Diebold U (2003) The surface of titanium dioxide. Surf Sci Rep 48:53–229
Ewald A, Glueckermann SK, Thull R, Gbureck U (2006) Antimicrobial titanium/silver PVD coatings on titanium. Biomed Eng 5:22
Forsgren J, Brohede U, Engqvist H, Strømme M (2011a) Co-loading of bisphosphonates and antibiotics to a biomimetic hydroxyapatite coating. Biotechnol Lett 33:1265–1268
Forsgren J, Brohede U, Piskounova S, Mihranyan A, Larsson S, Strømme M, Engqvist H (2011b) In vivo evaluation of functionalized biomimetic hydroxyapatite for local delivery of active agents. J Biomat Nanobiotech 2:149–154
Fox MA, Dulay MT (1993) Heterogenous photocatalysis. Chem Rev 93:341–357
Gallardo-Moreno AM, Pacha-Olivenza MA, Fernández-Calderón MC, Pérez-Giraldo C, Bruque JM, González-Martín ML (2010) Bactericidal behaviour of Ti6Al4 V surfaces after exposure to UV-C light. Biomaterials 31:5159–5168
Gamage J, Zhang Z (2010) Applications of photocatalytic disinfection. Int J Photoenergy 2010, Article ID 764870
Gonzalez RJ, Tarloff JB (2001) Evaluation of hepatic subcellular fractions for Alamar blue and MTT reductase activity. Toxicol In Vitro 15:257–259
Gristina AG (1994) Implant failure and the immuno-incompetent fibro-inflammatory zone. Clin Orthop Relat Res 298:106–118
Guinier A (1994) Diffraction by an imperfect crystals lattice in X-ray diffraction in crystals. Dover Publications Inc, New York, p 378
Heidenau F, Mittelmeier W, Detsch R, Haenle M, Stenzel F, Ziegler G (2005) Novel antibacterial titania coating: metal ion toxicity and in vitro surface colonization. J Mater Sci Mater Med 16:883–888
Hossain MF, Biswas S, Takahashi T, Kubota Y, Fujishima A (2008) Influence of direct current power on the photocatalytic activity of facing target sputtered TiO2 thin films. Thin Solid Films 517:1091–1095
Kasuga T, Kondo H, Nogami M (2002) Apatite formation on TiO2 in simulated body fluid. J Cryst Growth 235:235–240
Kleiman A, Marquez A (2007) Anatase TiO2 films obtained by cathodic arc deposition. Surf Coat Technol 201:6358–6362
Konstantinou IK, Sakellarides TM, Sakkas VA, Albanis TA (2001) Photocatalytic degradation of selected s-triazine herbicides and organophosphorus insecticides over aqueous TiO2 suspensions. Environ Sci Technol 35:398–405
Lavernia CJ, Drakeford MK, Tsao AK, Gittelsohn A, Krackow KA, Hungerford DS (1995) Revision and primary hip and knee arthroplasty—a cost analysis. Clin Orthop Relat Res 11:136–141
Lilja M, Genvad A, Åstrand M, Strømme M, Engqvist H (2011) Influence of microstructure and chemical composition of sputter deposited TiO2 thin films on in vitro bioactivity. J Mater Sci Mater Med 22:2727–2734
Lilja M, Welch K, Åstrand M, Engqvist H, Strømme M (2012) Effect of deposition parameters on the photocatalytic activity and bioactivity of TiO2 thin films deposited by vacuum arc on Ti–6Al–4V substrates. J Biomed Mater Res B 100B:1078–1085
Mahan J, Seligson D, Henry SL, Hynes P, Dobbins J (1991) Factors in pin tract infections. Orthopedics 14:305–308
Mihranyan A, Forsgren J, Strømme M, Engqvist H (2009) Assessing surface area evolution during biomimetic growth of hydroxyapatite coatings. Langmuir 25:1292–1295
Miyagi T, Kamei M, Mitsuhashi T, Ishigaki T, Yamazaki A (2004) Charge separation at the rutile/anatase interface: a dominant factor of photocatalytic activity. Chem Phys Lett 390(4–6):399–402
Navarro M, Michiardi A, Castaño O, Planell JA (2008) Biomaterials in orthopaedics. J R Soc Interface 5:1137–1158
Panda AB, Laha P, Harish K et al (2010) Study of bactericidal efficiency of magnetron sputtered TiO2 films deposited at varying oxygen partial pressure. Surf Coat Technol 205:1611–1617
Piskounova S, Forsgren J, Brohede U, Engqvist H, Strømme M (2009) In vitro characterization of bioactive titanium dioxide/hydroxyapatite surfaces functionalized with BMP-2. J Biomed Mater Res B 91B(2):780–787
Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J (2008) Periprosthetic joint infection: the incidence, timing and predisposing factors. Clin Orthop Relat Res 466:1710–1715
Ratkowsky DA, Olley J, McMeekin TA, Ball A (1981) Relationship between temperature and growth rate of bacterial cultures. J Bacteriol 149:1–5
Ratkowsky DA, Lowry RK, McMeekin TA, Chander RE (1983) Model for bacterial culture growth rate throughout the entire biokinetic temperature range. J Bacteriol 154:1222–1226
Riley DJ, Bavastrello V, Covani U, Barone A, Nicolini C (2005) An in vitro study of the sterilization of titanium dental implants using low intensity UV-radiation. Dent Mater 21:756–760
Rodriguez-Martinez JM, Pascual A (2006) Antimicrobial resistance in bacterial biofilms. Rev Med Microbiol 17:65–75
Sato T, Taya M (2006) Enhancement of phage inactivation using photocatalytic titanium dioxide particles with different crystalline structures. Biochem Eng J 28(3):303–308
Shiraishi K, Koseki H, Tsurumoto T, Baba K, Naito M, Nakayamac K, Shindoa H (2008) Antibacterial metal implant with a TiO2-conferred photocatalytic bactericidal effect against Staphylococcus aureus. Surf Interface Anal 41:17–22
Sirghi L, Hatanaka Y (2003) Hydrophilicity of amorphous TiO2 ultra-thin films. Surf Sci 530:323–327
Stewart PS, Costerton JW (2001) Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138
Temple J, Santy J (2004) Pin site care for preventing infections associated with external bone fixators and pins. Cochrane DB Syst Rev 1:CD004551
Uzunova-Bujnova M, Dimitrov D, Radev D, Bojinova A, Todorovsky D (2008) Effect of the mechanoactivation on the structure, sorption and photocatalytic properties of titanium dioxide. Mater Chem Phys 110:291–298
Welch K, Cai Y, Engqvist H, Strømme M (2010) Dental adhesives with bioactive and on-demand bactericidal properties. Dent Mater 26:491–499
Welch K, Cai Y, Strømme M (2012) A method for quantitative determination of biofilm viability. J Func Biomater 3:418–431
Zhao L, Chu PK, Zhang Y, Wu Z (2009) Review antibacterial coatings on titanium implants. J Biomed Mater Res 91B:470–480
Zhu L, Ye X, Tang G et al (2006) Corrosion test, cell behavior test, and in vivo study of gradient TiO2 layers produced by compound electrochemical oxidation. J Biomed Mater Res A 78:515–522
Acknowledgments
The Swedish Science Council, The Carl Trygger Foundation, The Göran Gustafsson Foundation, The Swedish Foundation for Strategic Research and Vinnova are acknowledged for financially supporting our research.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lilja, M., Forsgren, J., Welch, K. et al. Photocatalytic and antimicrobial properties of surgical implant coatings of titanium dioxide deposited though cathodic arc evaporation. Biotechnol Lett 34, 2299–2305 (2012). https://doi.org/10.1007/s10529-012-1040-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-1040-2