Abstract
The feasibility of using native lipase A from Candida antarctica (CAL-A) to esterify fatty acids with water-insoluble alcohols in the presence of excess water was investigated in stirred-tank reactors. For high reaction rates, a ratio of water:substrates of 0.6–1.4:1 (v/v) was required. CAL-A showed higher substrate selectivity for the esterification of saturated palmitic acid with branched-chain 2-ethyl-1-hexanol than for unsaturated oleic acid with linear alcohol (1-decanol). After 18 h at 70 °C in a 1.5 l bulk stirred-tank reactor, an 2-ethyl-1-hexyl palmitic acid ester was obtained near 100 % yield [molar ratio palmitic acid:2-ethyl-1-hexanol ~1:1.25, with 1.11 % (w/w) Novocor ADL (based on palmitic acid weight)].
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipases (EC 3.1.1.3) catalyze the hydrolysis of triacylglycerols (TAG) and, under certain conditions, carry out ester synthesis. In the presence of alcohols, fatty acid esters can be formed by two reactions: alcoholysis or esterification.
In alcoholysis, the glycerol component of TAG is substituted with monohydric water-soluble or water-insoluble alcohols. This reaction does not involve water as a reactant.
Esterification is the direct reaction of fatty acids with monohydric water-soluble or water-insoluble alcohols to form fatty acid esters. A number of lipases can catalyze this reaction. Esterification has been used in several reaction systems, with or without solvents, surfactants or immobilization of lipases (Yahya et al. 1998; Xu 2003; Christensen et al. 2003; Hills 2003).
The water content in the reaction medium is a significant factor. On the one hand, a minimal amount of water is necessary for the enzyme to be active. On the other, the formation of water causes a problem in the esterification reaction: in an aqueous medium the equilibrium of the fatty acid ester synthesis reaction is shifted in favour of hydrolysis. Thus, water has to be continuously removed from the reaction system in order to minimize the reverse hydrolysis reaction (Xu 2003).
In reactions where the substrates are poorly soluble in water and water is a product, the reaction yields in aqueous systems are generally low (Yahya et al. 1998). However in this paper, we describe a simple and convenient method to esterify fatty acids with a water-insoluble alcohol in presence of excess water and native CAL-A to produce fatty acid esters in stirred-tank reactors. To our knowledge, a similar lipase-catalyzed esterification process has not been reported.
When we tested the possibility of lipase A from Candida antarctica (CAL-A) to catalyze the hydrolysis of castor oil to produce ricinoleic acid, we found a surprising result: CAL-A has sufficient activity to catalyze the synthesis of ricinoleic acid estolides in aqueous environment. Consequently, on the basis of this result we tested the ability of native CAL-A to catalyze the esterification of fatty acids with water-insoluble alcohols in water-abundant systems.
Because of their high oxidation stability and the physical characteristics (density, viscosity, low-temperature performance) that are comparable to petroleum oil-based lubricants, 2-ethyl-1-hexyl palmitic acid ester plays an important role as non-polluting lubricant coolant (Wichmann and Bahadir 2007). Therefore, our investigations focused on the formation of this product.
For comparison, we additionally investigated the esterification of unsaturated oleic acid with the linear alcohol 1-decanol. 1-Decanol oleic acid ester (decyl oleate) is widely used in cosmetic formulations as it has characteristics similar to that of natural skin lipids (Hills 2003).
CAL-A has many applications (Kirk and Christensen 2002; de Maria et al. 2005; Liljeblad et al. 2010): it is stable in solutions as well as in immobilized form (Kirk and Christensen 2002). Its activity increases from 35 to 65–70 °C (Brenneis et al. 2004).
Its structure encompasses the features of hydrolases with the typical folds and the catalytic triad typical of lipases (Ericsson et al. 2008). There is a distinct lid domain characteristic of lipases but without apparent sequence homology to known lid structures (Ericsson et al. 2008; Liljeblad et al. 2010). CAL-A shows catalytic activation at the lipid–water interface (“interfacial activation”) (Martinelle et al. 1995; Liljeblad et al. 2010).
Materials and methods
Materials
All chemicals were from commercial suppliers. Novocor ADL from Novozymes A/S is a liquid preparation of a highly thermostable lipase from Candida antarctica, A-component (CAL-A) for non-food applications (Novozymes—enzyme description).
Apparatus and synthesis of fatty acid esters
Initial experiments were carried out in 100 ml cylindrical glass-tubes in which the components were stirred with a blade mixer at constant temperature in a water bath. The fatty acid component and the alcohol component were poured into a tube (with stirrer) and a solution of Novocor ADL and water into another tube. When the reaction temperature in both tubes was achieved, the lipase preparation (enzyme + water) was added to the substrate solution to start the enzymatic-catalyzed conversion. At intervals the mixer was stopped. The “ester phase” (top) was separated from the “water phase” (bottom) and was directly analysed by HPLC without any pre-treatment.
Further batch experiments were carried out in a 1.5 l bulk stirred-tank reactor. First, a solution of fatty acid and alcohol was prepared (70 °C). Next, the main bulk of water was added. Finally, when the reaction temperature was achieved, a solution of lipase and residual water was added. Progress of the esterification was followed by HPLC analysis.
HPLC analysis
The content of fatty acids as well as alkyl esters was determined on a standard column (C18) EC 250/4 Nucleosil 120-3 at 35 °C. Signals were detected by a diode array detector module and an evaporative light scattering detector.
Samples, ~30 mg, were diluted with 25 ml of the mobile phase acetonitrile/dichloromethane (90:10, v/v) and separated at 1.3 ml min−1. The elution scheme is shown in Table 1.
The Cass VP 5 software from Shimadzu was used for data analysis. The ester yield was calculated on the basis of the peak areas of residual fatty acids and fatty acid alkyl esters formed:
Yield = 100 [ester]/[fatty acid] + [ester], were [ ] are concentrations. Additionally, the residual fatty acids in the products were titrated with 0.1 M KOH in several instances.
Results and discussion
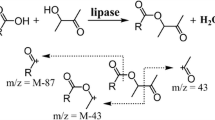
As exemplified in Fig. 1, CAL-A catalyzed the esterification of palmitic acid with 2-ethyl-1-hexanol in a water-abundant system.
To investigate the effect of water on the reaction rate, esterification reactions of palmitic acid with 2-ethyl-1-hexanol were carried out over a wide range of water contents. The results (see Fig. 2) are therefore novel as, with a high water content, one would expect the conditions to favour hydrolysis and lessen the extent of esterification.
Effect of water content on the yield of 2-ethyl-1-hexyl palmitic acid ester. Reaction mixture (equimolar): 19.9 g palmitic acid; 10.1 g 2-ethyl-1-hexanol; water as indicated; 0.4 g CAL-A; 100 ml stirred-tank reactor; 70 °C; 1,600 rpm; 120 min. Percentage of ester determined by HPLC as described in “Materials and methods” section
The system shows a plateau of high ester yield between 20 and 50 g water content. Esterification is slow in the presence of low quantities of water (“water/enzyme-in-substrates-emulsion”) or higher water contents (“substrates-in-water/enzyme-emulsion”). Such a change in the water-abundant reactant medium could influence access of the substrates to the active site of lipase because of the lower amount of interfacial area in such systems.
The highest conversions for 30 g of substrate were observed with 20–50 g water (equivalent to 20–50 ml water for 35 ml substrates). This means for high ester yields a volume ratio water:substrates of 0.6–1.4:1 is expedient. The large amount of interfacial area between the two phases (substrates—water/enzyme) achieved by intensive stirring is advantageous since CAL-A is activated upon adsorption at the interface. This adsorption promotes the opening of the lid that covers the active site to catalyze a lipid modification. Because of the various interactions between the substrates and the enzyme, a clear interpretation of the variations induced by physicochemical factors is difficult.
Considerable activity has been focused on the molecular basis of lipase reactions in order to understand and modify the preference of lipases for water.
There are several good reasons why lipases have a separate entrance to their active site for water. In hundreds of hydrolases, water tunnels have been detected by using molecular modelling (Larsen et al. 2010). Lipase B from Candida antarctica has a water tunnel that connects the solvent to the active site. Blocking this tunnel by site-directed mutagenesis decreased the relative amount of hydrolysis versus transesterification by approximately sixfold (Larsen et al. 2010).
Other lipases, however can use alternative mechanisms to activate or deactivate water.
Esterases/lipases favour hydrolysis, whereas acyltransferases favour transfer to an acceptor other than water. By comparison of X-ray structures a different oxyanion-loop orientation in the active site was identified. This structure mimics the transition state for the attack of water on the acyl-enzyme and shows a bridging water molecule between the carbonyl oxygen mentioned above and the sulfonyl oxygen that mimics the attacking water. A possible mechanistic role for this bridging water molecule is to position and activate the attacking water molecule in hydrolases, but to deactivate the attacking water molecule in acyl transferase (Jiang et al. 2011).
To understand the exceptional specificity of lipase A from Candida antarctica to favour esterification and alcoholysis (Brenneis et al. 2004) compared to hydrolysis more insights into its molecular structures are necessary. By all means, in the presence of 2-ethyl-1-hexanol and water CAL-A deactivates of its own accord the specific interaction between water and the active site to avoid hydrolysis.
Based on the results obtained in the 100 ml reactor, we carried out the production of 2-ethyl-1-hexyl palmitic acid ester in a 1.5 l batch stirred-tank reactor. An excess of alcohol was used to force the esterification reaction to the product. Figure 3 shows the progress of the esterification.
Time course for the esterification of palmitic acid with 2-ethyl-1-hexanol (a) and oleic acid with 1-decanol (b). Reaction mixtures (molar ratio ~ 1:1.25) a 450 g palmitic acid, 315 g 2-ethyl-1-hexanol, 750 g water, 5 g CAL-A; b 400 g oleic acid, 280 g 1-decanol, 700 g water, 5 g CAL-A. Reactions carried out in a 1.5 l stirred-tank reactor, 70 °C, 500 rpm. Percentage of ester determined by HPLC as described in “Materials and methods” section
The conversion was complete after 18 h (constant ester content in comparison with the ester contents of the next samples). After a separation time the “ester phase” (top) was discharged in a flask. Then the excess 2-ethyl-1-hexanol and traces of water were removed via vacuum distillation and the final ester product with an acid number of 2.88 was obtained. The residual water/enzyme mixture (including produced reaction water) in the stirred-tank reactor and the distilled alcohol can be re-used for the next batches.
The production of 1-decanol oleic acid ester is also shown in Fig. 3. This esterification was less efficient and is general in agreement with the results of Hesselbach et al. (2003) and Brenneis et al. (2004) on the same lipase but with different substrates. In this work, CAL-A catalyzed the alcoholysis reaction of saturated fatty acyl groups in triacylglycerols (TAG) with branched-chain alcohols better than with unsaturated acyl groups (in TAG) and linear alcohols. Therefore, further research will be focused on the question, whether the formation of fatty acid esters from TAG using CAL-A (Brenneis et al. 2004) is a real, single-stage process (alcoholysis) or a two-stage process (hydrolysis + esterification).
However, with regard to water content and the use of a native lipase, this esterification process is simple and robust to produce alkyl ester from palmitic acid and 2-ethyl-1-hexanol.
Conclusions
Candida antarctica lipase A catalyzes the esterification reactions of fatty acids with water-insoluble alcohols in presence of excess water. The enzyme has a preference for saturated fatty acids and the branched-chain alcohol 2-ethyl-1-hexanol. Thus, 2-ethyl-1-hexyl palmitic acid ester is produced at near 100 % yield in a water-abundant system.
References
Brenneis R, Baeck B, Kley G (2004) Alcoholysis of waste fats with 2-ethyl-1-hexanol using Candida antarctica lipase A in large-scale tests. Eur J Lipid Sci Technol 106:809–814
Christensen MW, Andersen L, Husum TL, Kirk O (2003) Industrial lipase immobilization. Eur J Lipid Sci Technol 105:318–321
de Maria PD, Carboni-Oerlemans C, Tuin B, Bargeman G, van der Meer A, van Gemert R (2005) Biotechnological applications of Candida antarctica lipase A: state-of-the-art. J Mol Catal B Enzyme 37:36–46
Ericsson DJ, Kasrayan K, Johansson P, Bergfors T, Sandström AG, Bäckvall JE, Mowbray SL (2008) X-ray structure of Candida antarctica Lipase A shows a novel lid structure and a likely mode of interfacial activation. J Mol Biol 376:109–119
Hesselbach J, Herrmann C, Bock R, Dettmer T, Kley G, Köcher P, Brenneis R, Meyer-Pittroff R, Falk O, Hansen A (2003) Kühlschmierstoffe aus technischen tierischen Fetten und Altspeisefetten—Herstellung, Technologie und Ökobilanzierung. Transkript (Sonderheft, Nachhaltige Biokatalyse, Okt. 2003), pp 24–27
Hills G (2003) Industrial use of lipases to produce fatty acid esters. Eur J Lipid Sci Technol 105:601–607
Jiang Y, Morley KL, Schrag JD, Kazlauskas RJ (2011) Different active-site loop orientation in serine hydrolases versus acyltransferases. Chem Bio Chem 12:768–776
Kirk O, Christensen MW (2002) Lipases from Candida antarctica: unique biocatalysts from a unique origin. Org Process Res Dev 6:446–451
Larsen MW, Zielinska DF, Martinelle M, Hidalgo A, Jensen LJ, Bornscheuer UT, Hult K (2010) Suppression of water as a nucleophile in Candida antarctica lipase B catalysis. Chem Bio Chem 11:796–801
Liljeblad A, Kallio P, Vainio M, Niemi J, Kanerva LT (2010) Formation and hydrolysis of amide bonds by lipase A from Candida antarctica: exceptional features. Org Biomol Chem 8:886–895
Martinelle M, Holmquist M, Hult K (1995) On the interfacial activation of Candida antarctica lipase A and B as compared with Humicola lanuginosa lipase. Biochim Biophys Acta 1258:272–276
Wichmann H, Bahadir M (2007) Bio-based ester oils for use as lubricants in metal working. Clean 35:49–51
Xu X (2003) Engineering of enzymatic reactions and reactors for lipid modification and synthesis. Eur J Lipid Sci Technol 105:289–304
Yahya ARM, Anderson WA, Moo-Young M (1998) Ester synthesis in lipase-catalyzed reactions. Enzyme Microb Technol 23:438–450
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brenneis, R., Baeck, B. Esterification of fatty acids using Candida antarctica lipase A in water-abundant systems. Biotechnol Lett 34, 1459–1463 (2012). https://doi.org/10.1007/s10529-012-0928-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-0928-1