Abstract
Single-stranded DNA (ssDNA) aptamers against four organophosphorus pesticides (phorate, profenofos, isocarbophos and omethoate) were simultaneously isolated from an immobilized random ssDNA library by systematic evolution of ligands by exponential enrichment (SELEX) technique. After 12 rounds of in vitro selection, five ssDNA aptamer candidates were selected and their binding affinities were identified by a novel method using a molecular beacon. Two of the five ssDNA sequences, SS2-55 and SS4-54, demonstrated higher affinities and specificities to the four organophosphorus pesticides. They were defined as broad-spectrum aptamers binding to four different targets and their simulated secondary structures showed highly distinct features with typical stem and loop structures. The dissociation constant of SS2-55 and SS4-54 binding to the four organophosphorus pesticides ranged from 0.8 to 2.5 μM. These aptamers offered application potential in the analysis and/or neutralization of the residues of the four organophosphorus pesticides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aptamers are single-stranded DNA (ssDNA) or RNA that can bind to various target molecules with high affinity and specificity. They are emerging as a new biosensor to be applied in analytical systems due to their appealing characteristics such as, highly synthesizable, easy to be structurally modified, highly water-soluble, biocompatible, and reasonably stable (Tombelli et al. 2005; Mascini 2008). Aptamers are superior to antibodies because of their broader range of application. They are able to bind not only to proteins (Tang et al. 2007; Hasegawa et al. 2008; Tsukakoshi et al. 2010) but also to other molecules, including metal ions (Kawakami et al. 2000), and small molecules (Mann et al. 2005; Niazi et al. 2008; Cruz-Aguado and Penner 2008; Kim et al. 2010; Mehta et al. 2011).
Organophosphorus pesticides, including phorate, profenofos, isocarbophos and omethoateas, are highly poisonous pesticides and are still used in some agricultural practices. Any possible residues could cause serious and permanent consequences to human health in general and in children’s health in particular. Therefore, there is an urgent need to develop reagents that can detect and neutralize the residues of organophosphorus pesticides. To the best of our knowledge, there is no report that aptamers have been investigated for this purpose. The primary aim of this paper is to select aptamers that bind to organophosphorus pesticides, and characterize their affinity and specificity.
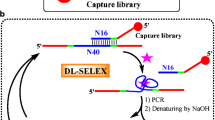
Aptamers have been randomly selected from sequence pools by using the systematic evolution of ligands by exponential enrichment (SELEX) in vitro (Tuerk and Gold 1990; Ellington and Szostak 1990). Conventional SELEX process is limited to select aptamers that bind to single targe molecules. The secondary purpose of this study is to apply a modified SELEX that can specifically select broad-spectrum aptamers which can simultaneously bind to four organophosphorus pesticides from an immobilized random ssDNA library. This technique would improve the efficiency of aptamer selection.
Materials and methods
Materials
All the pesticides were purchased from Dr. Ehrensorfer GmbH. All the oligonucleotides were synthesized by Generay, and purified by PAGE. ssDNA library immobilization and ssDNA were prepared by using Thermo-Pierce streptavidin agarose resins.
Immobilization of ssDNA library
A random ssDNA library (5′-CCTGCCACGCTCCGCAAGCTT-N10-CTGCAGCGATTCTTGATCG-N20-TAAGCTTGCACCCGCATCGT-3′) consisting of one constant sequence in the middle and of primer binding sequences at both ends was used. The constant sequence was used to immobilize the ssDNA library by a partly complementary sequence (B-B: 5′-biotin-TACCGCAAAAAAAAACAAGAATCGCTGCAG-3′). The primer binding sequences were hybridized by two complementary sequences (P1: 5’-GCGGAGCGTGGCAGG-3′, P2: 5′-ACGATGCGGGTGCCAAGCTTA-3′) while the ssDNA library was immobilized. Before each round of selection, the ssDNA library, B-B, P1 and P2 were denatured at 90°C for 3 min in the selection buffer (300 mM NaCl, 50 mM KCl, 10 mM MgCl2, 50 mM Tris/HCl, and pH 8.3) and annealed for 30 min at room temperature. This mixture was then incubated with 200 μl streptavidin/agarose resins (pre-washed five times with the selection buffer) in a filtration column for 40 min at room temperature in order to immobilize the ssDNA library.
Selection of ssDNA
In vitro selection was performed using a previously reported method (Nutiu and Li 2005) with modifications. A mixture (200 μl) of organophosphorus pesticides at 0.5 mM were added to the immobilized ssDNA library filtration column, and incubated for 40 min. Following centrifugation (600 × g, 1 min, 4°C), the ssDNAs in the eluted filtrate were amplified by PCR with a biotin-5′-labeled primer (P2-B: 5′-biotin ACGATGCGGGTGCCAACTT-3′) and an FAM-5′-labeled primer (F-P3: 5′-FAM-CCTGCCACGCTCCGC-3′). The PCR products were incubated with 200 μl streptavidin/agarose resins for 40 min at room temperature, and the FAM-labeled sense strand was released by addition of 0.1 M NaOH. The ssDNAs were precipitated by ethanol and collected with glycogen for the next round of selection. After 12 rounds of selection, the selected ssDNAs were amplified with a non-modified primer (P3: 5′-CCTGCCACGCTCCGC-3′) before amplified DNAs were cloned and sequenced.
Binding affinity assay of ssDNA
A molecular beacon (5′-FAM-CTGCACAAGAATCGCTGCAG-DABCYL-3′) was designed, which was partly complementary with the middle constant nucleotides-sequence of the selected ssDNAs. A competitive inhibition method (Fig. 1) was developed and applied to the binding assay of the selected ssDNAs. The selection buffer, or target molecules solution, and the molecular beacon were mixed with the selected ssDNAs. After the mixture had been incubated for 50 min at room temperature, its fluorescence intensity was measured at excitation wavelength at 485 nm and emission wavelength at 535 nm. The inhibition ratios were calculated by the following equation:
where R was the inhibition ratio, C was the fluorescence intensity of the control group, T was the fluorescence intensity of the target molecules group and B was the fluorescence intensity of the molecular beacon. The inhibition ratio indicated the affinity and specificity of the selected ssDNAs.
Mechanism of competitive inhibition method. a Secondary structure of the molecular beacon was predicted to be a hairpin structure. b The background fluorescence of the molecular beacon was very low because of fluorescence resonance energy transferring. The fluorescence intensity was increased when the molecular beacon binds to the selected ssDNA, and inhibited while organophosphorus pesticides competitively bind to aptamers
Determination of dissociation constant (Kd)
An FAM-5′-labeled sequence (F-P4: 5′-FAM-CAAGAATCGCTGCAG) that partly complementary with the middle constant nucleotides-sequence of the selected aptamers was used to calculate Kd. Aptamers at 200 nM were incubated with pesticides from 100 nM to 2 μM for 30 min at room temperature. Then, F-P4 at 200 nM was added to the solution, and incubated for 30 min. Those free aptamers, which did not bind to the pesticides, would bind to the F-P4 at this time. The unbound F-P4 was separated with a 10 kDa filtration column. The concentrations of aptamer-complexes and unbound pesticides were estimated by the fluorescence intensity of unbound F-P4. The Kd was calculated using the formula reported previously (Wang et al. 2000):
the binding ratio B/F, where B was the concentration of aptamer-complex, and F was the concentration of free pesticide. The value of Kd was determined by Scatchard plot analysis.
Results
Selection of DNA aptamers
DNA aptamers binding to phorate, profenofos, benzoate and omethoate were selected simultaneously from an immobilized random ssDNA library consisting of 1014–1015 nucleotides. During each round of selection, the ssDNAs binding to the organophosphorus pesticides were eluted from the immobilized ssDNA library, and enriched as the selection round progressed. In the first round, the all eluted filtrate was amplified by PCR to ensure the capacity of the ssDNA library. From the second round, the ratio of fluorescence intensity of the eluted solution to that of the second ssDNA library was determined by the output/input ratio (Fig. 2). In the second round, the output/input was ~1.3%. This ratio was positively correlated with the number of selection rounds. At the twelfth selection round, the output/input ratio was maximal at 41%.
Efficiency of each round of selection. From the second round, the fluorescence intensity of the secondary library and the eluted filtrate were measured on a black 96-well flat bottom assay plate (Costar 3915) using Mithras microplate reader (LB 940; Berthold) at excitation wavelength 485 nm and emission wavelength 535 nm. The selection efficiency was determined by the ratio between fluorescence intensity of the eluted filtrate and that in the solution of the second library
Therefore, the eluted ssDNAs of the twelfth selection were amplified with non-modified primer and cloned into a pUC-T simple vector by using a pUC-T simple cloning kit. Twenty white colonies were identified and picked. Fifteen positive colonies with plasmids containing inserted ssDNA were sequenced. Top five ssDNAs with highest homogeneity in sequence are shown in Table 1, and selected for further characterization.
Characterization of the selected ssDNAs
To determine the affinity of the five selected ssDNAs, binding assay was performed by the competitive inhibition. The inhibition ratios of every pesticide are shown in Fig. 3. Because the inhibition ratio was positively correlated with the binding affinity of the selected ssDNAs, SS2-55 and SS4-54 demonstrated stronger binding affinity to the four organophosphorus pesticides than did SS3-53, SS9-55 and SS13-55. In binding to the four target molecules, SS2-55 and SS4-54 displayed the highest activity in binding to isocarbophos with inhibition ratios of over 75%, and their activity in binding to the other three target molecules was also sufficiently high with the inhibition ratios of above 39%. These results suggested that SS2-55 and SS4-54 might be broad-spectrum aptamers.
Affinity of the selected ssDNAs. The four organophosphorus pesticides at final concentration of 500 μM and the molecular beacon at final concentration of 125 nM were mixed with the five selected ssDNAs at final concentration of 100 nM, and incubated for 50 min at room temperature before inhibition ratio was calculated
The secondary structures of SS2-55 and SS4-54 aptamers were predicted by DNAMAN software, and typical stem and loop motifs are shown in Fig. 4. The design of binding domain of aptamers to their targets was based on structural compatibility, aromatic rings stacking, electrostatic and van der Waals interactions, and hydrogen bonding, or from a combination of these requirements (Stoltenburg et al. 2007). The structures of most selected aptamers are typically assembled by stems, loops, bulges, hairpins, triplexes, pseudoknots, or quadruplexes. Therefore, loops 2-1, 2-2, 2-3, 2-4, 4-1, 4-2 and 4-3 are most likely active sites of the two aptamers binding to the four organophosphorus pesticides.
The specificities of SS2-55 and SS4-54 aptamers to nine structurally similar pesticides of phorate, profenofos, isocarbophos and omethoate, including imidacloprid, acetamiprid, chlorpyrifos, methamidophos, dichlorvos, parathion, dimethoate, monocrotophos and phoxim was also examined and the results are shown in Fig. 5. The inhibition ratios of the nine pesticides were below 15%, and SS2-55 and SS4-54 displayed weaker affinities with the above nine pesticides. These results suggested that the SS2-55 and SS4-54 aptamers could bind to phorate, profenofos, isocarbophos and omethoate with higher specificity.
Specificity of SS2-55 and SS4-54 aptamers. Imidacloprid, acetamiprid, chlorpyrifos, methamidophos, dichlorvos, parathion, dimethoate, monocrotophos and phoxim at 500 μM and the molecular beacon at 125 nM were mixed with SS2-55 and SS4-54 aptamers at 100 nM, and incubated for 50 min at room temperature before the inhibition ratio was calculated
Determination of Kd
The above results of binding affinity assay suggested that SS2-55 and SS4-54 aptamers had greater affinity to the four organophosphorus pesticides than that of other three ssDNAs. Thus, the equilibrium Kd of SS2-55 and SS4-54 aptamers to organophosphorus pesticides were measured by a fluorescent-combining, equilibrium filtration method. The Kd by Scatchard plot analysis are shown in Table 2. The dissociation constants of SS2-55 and SS4-54 binding to the four organophosphorus pesticides ranged from 0.8 to 2.5 μM.
Discussion
In classical procedures of small molecules aptamers selection, target molecules have been immobilized on Sepharose or magnetic beads before being incubated with the oligonucleotide library for binding. In reality, most of the small molecule targets were not directly immobilized on the matrix, and complicated chemical modification was required to introduce an active group (Vianini et al. 2001; Niazi et al. 2008). Traditional aptamer selection technique is also limited by its capacity to single target molecule. In this report, we have introduced a new method that selects broad-spectrum aptamers from an immobilized random ssDNA library consisting of 1014–1015 nucleotides with the capacity of an individual aptamer binding to four organophosphorus pesticides. This novel method applies a molecular beacon to identify the selected ssDNAs. During validation of this technique, we selected SS2-55 and SS4-54 aptamers. They equipped with a broad-spectrum to bind four organophosphorus pesticides with high binding affinity and specificity.
The Kd for interaction between small molecule targets and aptamers is usually calculated by the equilibrium filtration method. The organophosphorus pesticides were, however, intensively adsorbed by the filtration column resulting in 60–80% of organophosphorus pesticides being unrecoverable. To overcome this adsorption, we applied a fluorescent-combining, equilibrium filtration method to indirectly estimated Kd. Using this method, we have successfully obtained the Kd of SS2-55 and SS4-54 aptamers.
In conclusion, this is the first report that two DNA aptamers have been selected for potential application in the analysis and/or neutralization of the residues of the four organophosphorus pesticides.
References
Cruz-Aguado JA, Penner G (2008) Determination of ochratoxin A with a DNA aptamer. J Agric Food Chem 56:10456–10461
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822
Hasegawa H, Sode K, Ikebukuro K (2008) Selection of DNA aptamers against VEGF165 using a protein competitor and the aptamer blotting method. Biotechnol Lett 30:829–834
Kawakami J, Imanaka H, Yokota Y, Sugimoto N (2000) In vitro selection of aptamers that act with Zn2+. J Inorg Biochem 82:197–206
Kim YS, Hyun CJ, Kim IA, Gu MB (2010) Isolation and characterization of enantioselective DNA aptamers for ibuprofen. Bioorg Med Chem 18:3467–3473
Mann D, Reinemann C, Stoltenburg R, Strehlitz B (2005) In vitro selection of DNA aptamers binding ethanolamine. Biochem Biophys Res Commun 338:1928–1934
Mascini M (2008) Aptamers and their applications. Anal Bioanal Chem 390:987–988
Mehta J, Dorst BV, Rouah-Martin E, Herrebout W, Scippo ML, Blust R, Robbens J (2011) In vitro selection and characterization of DNA aptamers recognizing chloramphenicol. J Biotechnol 155:361–369
Niazi JH, Lee SJ, Kim YS, Gu MB (2008) ssDNA aptamers that selectively bind oxytetracycline. Bioorg Med Chem 16:1254–1261
Nutiu R, Li Y (2005) In vitro selection of structure-switching signaling aptamers. Angew Chem Int Ed Engl 117:1085–1089
Stoltenburg R, Reinemann C, Strehlitz B (2007) SELEX-A (r) evolutionary method to generate high-affinity nucleic acid ligands. Biomol Eng 24:381–403
Tang J, Yu T, Guo L, Xie J, Shao N, He Z (2007) In vitro selection of DNA aptamer against abrin toxin and aptamer-based abrin direct detection. Biosens Bioelectron 22:2456–2463
Tombelli S, Minunni M, Mascini M (2005) Analytical applications of aptamers. Biosens Bioelectron 20:2424–2434
Tsukakoshi K, Harada R, Sode K, Ikebukuro K (2010) Screening of DNA aptamer which binds to α-synuclein. Biotechnol Lett 32:643–648
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510
Vianini E, Palumbo M, Gatto B (2001) In vitro selection of DNA aptamers that bind L-Tyrosinamide. Bioorg Med Chem 9:2543–2548
Wang Q, Wang Y, Luo G (2000) Determination of the binding constant between progesterone and its monoclonal antibody using affinity capillary electrophoresis. Chin J Anal Chem 28:731–734
Acknowledgments
This work was supported by research grants received from the National Natural Science Foundation of China (30871658), the “948” Projects of Chinese Agriculture Ministry (2011-Z46 and 2011-G5-7), the Independent Innovation Foundation of Jiangsu Province in China [CX (10)236], the Jingsu Planed Projects for Postdoctoral Research Funds (0802023B) and Suzhou Key Technology Research and Development Program (SN201129).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Liu, X., Zhang, Q. et al. Selection of DNA aptamers that bind to four organophosphorus pesticides. Biotechnol Lett 34, 869–874 (2012). https://doi.org/10.1007/s10529-012-0850-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-0850-6