Abstract
Hairy root cultures of Salvia tomentosa were initiated by transformation with Agrobacterium rhizogenes. To prevent necrosis in the explants and to protect young hairy roots, Amberlite XAD-4 resin, in combination with a temporary immersion cultivation system, was applied. HPLC analyzes showed that the resin adsorbed more than 93% of the released phenolic acids and 100% of the released flavonoids. The decreased content of the released phenolics significantly reduced their destructive effects on the plant tissues, prevented, and speeded up the appearance of hairy roots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Balsamic sage (Salvia tomentosa Mill.) is widely used either for tea preparation or as flavoring agent in perfumery and cosmetics. The aerial parts of mature plant accumulate essential oils and produce various compounds with strong antimicrobial and antioxidant activities (Tepe et al. 2005). In Bulgaria this plant is under threat and spreads in very limited populations. Since 2007 its collection has been prohibited for commercial purposes. Plant in vitro systems are promising technologies for obtaining biologically active substances and also for basic research of molecular biology and biochemistry of plant cells. Secondary metabolites are defined as products of differentiation and for this reason, research has been done on in vitro cultures of hairy roots derived from differentiated cells (Georgiev et al. 2007; Veena and Taylor 2007). However, there are no reports for hairy root cultures initiation of by Salvia tomentosa Mill. Probably the main reason is due to the presence of highly active molecules in the tissues, inhibiting the growth of bacteria and/or new formed hairy roots.
In the present work we describe an effective protocol for hairy roots obtaining of Salvia tomentosa Mill. mature plants using two-phase temporary immersion system for Agrobacterium rhizogenes transformation.
Materials and methods
Plant material and explants sterilization
Young leaves of naturally growing mature Salvia tomentosa Mill. plants were harvested in an experimental field of the Institute of Botany, Bulgarian Academy of Sciences, near Sofia, Bulgaria. They were sterilized by 70% (v/v) ethanol for 20 s followed by treatment with either calcium hypochlorite [4, 6 and 8% (w/v)] or Domestos [10, 20 and 30% (v/v)] for 3, 6 or 9 min.
Transformation techniques
Classical transformation approach: For hairy roots, suspensions of Agrobacterium rhizogenes ATCC 15834 were used with OD600 0.5, 0.75 or 1. The methods of direct infection and co-cultivation were applied (Georgiev et al. 2007). 72 h after transformation, explants were transferred to solid MS media + 100, 200, or 400 mg Cefotaxime/l to kill the bacteria. Half of the explants were cultivated in darkness, and the remainder with illumination (16 h light and 8 h dark).
Submerged two-phase system for Agrobacterium rhizogenes transformation: Co-cultivation with A. rhizogenes ATCC 15834 (OD600 = 0.75) was used. After 72 h, the explants were transferred into flasks with 100 ml liquid MS media + 200 mg Cefotaxime/l and 1 g Amberlite XAD-4 resin, held in a net. Cultivation was at 26°C with shaking (110 rpm) and illumination (16 h light, 8 h dark).
Two-phase temporary immersion system for Agrobacterium rhizogenes transformation: Co-cultivation with A. rhizogenes ATCC 15834 (OD600 = 0.75) was used. After 72 h, the explants were transferred into a RITA apparatus (CIRAD Ltd., France) with 200 ml MS medium + 200 mg Cefotaxime/l and 2 g Amberlite XAD-4 resin in a net. Cultivation was at 26°C, with illumination (16 h light, 8 h dark), at immersion frequencies of 15 min flooding and 4, 8, and 12 h stand-by periods, respectively. The inlet air during flooding periods was AT 60 l/h for each RITA apparatus.
Amberlite XAD-4 activation
The activation was carried out according to the manufacturer’s instructions. The activated resin was washed with double distillated water to neutral pH. The packs were sterilized in liquid MS medium at 121°C for 30 min before using in the experiments.
Extraction of phenolics
From the culture liquid: culture media were evaporated to dryness at 60°C, and the residue was dissolved in 10 ml methanol, cooled at −20°C for 3 h and the supernatant was passed through a 0.22 μm filter.
From Amberlite XAD-4 resin: the resin was extracted with 200 ml methanol for 2 h on a shaker in triplicate. The extracts were evaporated to dryness and the residue was dissolved in 10 ml methanol, filtered through 0.22 μm filter.
Analysis
Total phenolics: Total phenolics were assayed by the Folin–Ciocalteu method with gallic acid as standard (Georgiev et al. 2010).
HPLC: Samples were analysed for phenolic acids and flavonoids using Supelco Discovery HS C18 column (25 cm × 4.6 mm, 5 μm) at 25°C. Details given in Table 1.
PCR analysis
Integration of the Ri T-DNA into the plant genome was confirmed by PCR. Genomic DNA from isolated bacteria-free hairy root lines, the intact plant leaves, as well as from A. rhizogenes ATCC 15834 was extracted using InnuPREP Plant DNA kit (Analytik-Jena, Germany) and quantified by NanoDrop 2000C spectrophotometer (Thermo-scientific, USA). PCR was performed in 25 μl with 80 ng gDNA, 2 μM of each rolA gene-specific primer : forward 5′-AGA ATG GAA TTA GCC GGA CTA-3′ and reverse 5′-GTA TTA ATC CCG TAG GTT TGT TT-3′, 200 μM of each nucleotide, 1% reaction buffer (10 mM Tris/HCl, pH 8.3; 50 mM KCl, 1.5 mM MgCl2) and 0.5 U Taq DNA polymerase per reaction-Ready-to go PCR Analysis Beads kit (GE Healthcare, USA), in Quanta Biotech PCR system QB-24 thermal cycler. PCR was carried out using an initial denaturation at 94°C for 5 min followed by 35 cycles each of 1 min denaturation at 94°C, 1 min annealing at 55°C and 1 min extension at 72°C with a final extension of 72°C for 10 min. PCR products were separated on a 1.5% agarose gel electrophoresis in 0.6% TBE buffer [0.09 M TrisBoric acid and 2 mM EDTA (pH 8.0)] at 90 V, visualized by staining with ethidium bromide (10 mg/ml) and documented using a gel documentation system (BioDoc-it, UVP, USA). PCR products were determined by comparison to external size standard (50–2000 bp), USB Corporation, USA.
Results and discussion
Sterilization of the plant tissues prior to transformation with Agrobacterium was most effective with 6% (w/v) Ca(ClO)2 for 6 min. More than 90% of leaves remained viable and were free of contaminants.
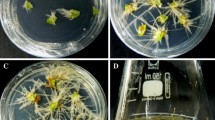
The effective removal of the Agrobacterium and the best viability of explants were achieved at cultivation with illumination on MS medium + 200 mg Cefotaxime/l. However, leakages of brown sap from the wounding points were observed and at the end of the third week plant tissues became dark brown (Fig. 1a, b). Under these conditions, root tips were observed at the infection points. Obviously, the A. rhizogenes ATCC 15834 had clearly transformed S. tormenotsa explants but the formed young hairy roots were killed by the release of necrotic tissue compounds (Fig. 1c). Most of these compounds possess significant phytotoxic activity (Reigosa and Pazos-Malvido 2007) or have strong allelopathic effects by inhibiting the plant root growth (Makoi and Ndakidemi 2007).
Explants of Salvia tomentosa Mill. plants, infected with Agrobacterium rhizogenes ATTC 15834: one day after infection (a), three weeks after infection (b); Dead hairy roots on necrotic leaves, cultivated for three weeks on solid MS medium (c). Explants with hairy roots, cultivated in flasks with Amberlite XAD-4 for four weeks (d); Two weeks old explants and hairy roots, cultivated in two-phase temporary immersion systems at 15 min flooding and 12 h stand-by periods (e); PCR amplification of rolA gene (f): DNA marker (1), the extracted DNA from the leaf of the intact plant Salvia tomentosa Mill. (2), the extracted DNA from the hairy roots of the transformed Salvia tomentosa Mill. (3), the amplified DNA of rolA gene from Agrobacterium rhizogenes ATTC 15834 plasmid (4)
To overcome this problem, a two-phase cultivation system was applied. Such systems were widely used for target product recovery in various plant in vitro biotechnologies (Park et al. 2011). The application of this technique allowed us to remove the phenolics from the cultivation medium, to prevent the explants and the formed young hairy roots from their destructive effects. Recently, the polymeric resin Amberlite XAD-4 was successfully applied as adsorbent of the released toxic phenolics in the selection procedure for regeneration of transgenic Aloe vera plants (Velcheva et al. 2010). Explants cultivated with this resin remained fresh, without necrosis, and 50% of them formed hairy roots at the end of forth week (Fig. 1d). The analyses of the secreted phenolics showed that 73% of them were adsorbed from the resin. However, in spite of the effective explants protection and the successful hairy root initiation, this approach had a serious disadvantage, because developed hairy roots were easily broken to small pieces by the mechanical interactions of the explants and resin nets.
For solving the problem, a temporary immersion cultivation system was applied. These cultivation systems were developed mainly for the needs of plants micropropagation (Debnath 2009) but, recently, they found application in transgenic plant regeneration (Hanhineva and Karenlampi 2007) and secondary metabolite production (Pavlov and Bley 2006; Ivanov et al. 2011). Their suitable design allowed an effective separation of the resin nets from the explants. Further, it was established that the best cultivation regime was at 15 min flooding and 12 h stand-by periods. Under these conditions, 100% of the explants intensively formed fast growing hairy roots at the end of the second week. The explants remained green and transformed roots were white, branched and possessed intensive growth (Fig. 1e). The positive effect on the hairy roots formation was due to the depletion of the phenolic compounds. HPLC analyses (Table 2) showed that explants of S. tomentosa secreted high concentrations of phenolic acids and flavonoids, as most of them were adsorbed from the resin. These results are important because phenolic acids above 1000 μg/l have strong growth inhibitory effects on plants roots and seedlings (Einhellig et al. 1985; Machrafi et al. 2006; Makoi and Ndakidemi 2007; Reigosa and Pazos-Malvido 2007). Moreover, some compounds, such as salicylic acid, vanillic acid, caffeic acid, p-coumaric acid and ferulic acid, have root growth inhibitory effect depending of their concentration (Mandal et al. 2010). 85% of these compounds were removed from the medium. Clorogenic acid, having strong prooxidant nature (Sakihama et al. 2002), was also removed (93%) from the medium. In contrast, only 39% of gallic acid, which acts primary as antioxidant (Mandal et al. 2010), was absorbed from the resin.
Flavonoids were completely adsorbed from the Amberlite XAD-4 resin (Table 2). Removing such active allelopathic compounds, with strong toxic and growth inhibitory actions, as myricetin, hesperidin and quercetin (Einhellig et al. 1985; Inderjit and Dakshini 1991; Makoi and Ndakidemi 2007), from the cultivation medium, provided optimal conditions for developing young hairy roots. Absent of flavonoids in the culture medium probably was the main reason for shorter period of hairy roots appearance (only two weeks after transformation in comparison with the four weeks at the submerged cultivation in flasks).
The successful transformation of obtained hairy roots was proved by PCR amplification of rolA gene (Fig. 1f). The confirmed transfer of the rolA gene into the transformed S. tomentosa hairy root line was clear proof for its successful genetic transformation (Rahimi et al. 2008).
In conclusion, for the first time we reported successful Agrobacterium rhizogenes transformation of plant tissues based on combined application of an adsorbent resin and a temporary immersion cultivation system. This approach is overcoming the negative effects of the secreted phenolic compounds with antimicrobial and phytotoxic activities. The developed protocol is essentially suitable for transformation of plants biosynthesizing huge amounts of phenolic compounds. Following the described procedure, 111 hairy root lines of Salvia tomentosa Mill. were obtained. After six months of cultivation (subcultured every 25 days), 35% of them (38 lines) showed stable growth and morphological characteristics. Further these hairy roots will be evaluated for bioactive secondary metabolites production.
References
Debnath SC (2009) Characteristic of strawberry plants propagated by in vitro bioreactor culture and ex vitro propagation method. Eng Life Sci 9(3):239–246
Einhellig FA, Leather GR, Hobbs LL (1985) Use of Lemna minor L. as a bioassay in allelopathy. J Chem Ecol 11:65–72
Georgiev MI, Pavlov AI, Bley T (2007) Hairy root type plant in vitro systems as sources of bioactive substances. Appl Microbiol Biotechnol 74:1175–1185
Georgiev VG, Weber J, Kneschke EM, Denev PN, Bley T, Pavlov AI (2010) Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot Beta vulgaris cv. Detroit dark red. Plant Foods Hum Nutr 65:105–111
Hanhineva KJ, Karenlampi SO (2007) Production of transgenic strawberries by temporary immersion bioreactor system and verification by TAIL-PCR. BMC Biotechnol 7:11–22
Inderjit, Dakshini KMM (1991) Hesperetin 7-rutinoside (hesperidin) and taxifolin 3-arabinoside as germination and growth inhibitors in soils associated with the weed, Pluchea lanceolata (DC) C.B. Clarke (Asteraceae). J Chem Ecol 17:1585–1591
Ivanov I, Georgiev V, Georgiev M, Ilieva M, Pavlov A (2011) Galanthamine and related alkaloids production by Leucojum aestivum L. shoot culture using a temporary immersion technology. Appl Biochem Biotechnol 163(2):268–277
Machrafi Y, Prevost D, Beauchamp CJ (2006) Toxicity of phenolic compounds extracted from bark residues of different ages. J Chem Ecol 32:2595–2615
Makoi JHJR, Ndakidemi PA (2007) Biological, ecological and agronomic significance of plant phenolic compounds in rhizosphere of the symbiotic legumes. Afr J Biotechnol 6:1358–1368
Mandal SM, Chakraborty D, Dey S (2010) Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav 5:359–368
Park NI, Tuan PA, Li X, Kim YK, Yang TJ, Park SU (2011) An efficient protocol for genetic transformation of Platycodon grandiflorum with Agrobacterium rhizogenes. Mol Biol Rep 38(4):2307–2313. doi:10.1007/s11033-010-0363-0
Pavlov A, Bley T (2006) Betalains biosynthesis by Beta vulgaris L. hairy root culture in a temporary immersion cultivation system. Proc Biochem 41:848–852
Rahimi K, Haghbeen K, Marefatjo J, Jazii FR, Sheikhani R (2008) Successful production of hairy root of Valeriana sisymbriifolium by Agrobacterium rhizogenes. Biotechnology 7:200–204. doi:10.3923/biotech.2008.200.204
Reigosa MJ, Pazos-Malvido E (2007) Phytotoxic effects of 21 plant secondary metabolites on Arabidopsis thaliana germination and root growth. J Chem Ecol 33:1456–1466
Sakihama Y, Cohen MF, Grace SC, Yamasaki H (2002) Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology 177:67–80
Tepe B, Daferera D, Sokmen A, Sokmen M, Polissiou M (2005) Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem 90:333–340
Veena V, Taylor CG (2007) Agrobacterium rhizogenes: recent developments and promising applications. In Vitro Cell Dev Biol Plant 43:383–403
Velcheva M, Faltin Z, Vardi A, Hanania U, Eshdat Y, Dgani O, Sahar N, Perl A (2010) Aloe vera transformation: the role of Amberlite XAD-4 resin and antioxidants during selection and regeneration. In Vitro Cell Dev Biol Plant 46:477–484
Acknowledgments
This research was supported by the Bulgarian Science Foundation, Bulgarian Ministry of Education and Science (projects DMU – 02/9, 2009 and DNTS 02/5 2010), and European Social Fund under Human Resources Development Operational Program (project BG051PO001-3.3.04/32).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marchev, A., Georgiev, V., Ivanov, I. et al. Two-phase temporary immersion system for Agrobacterium rhizogenes genetic transformation of sage (Salvia tomentosa Mill.). Biotechnol Lett 33, 1873–1878 (2011). https://doi.org/10.1007/s10529-011-0625-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0625-5