Abstract
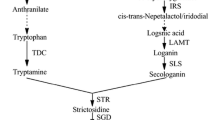
Silymarin (Sm) from the fruit of Silybum marianum is an isomeric mixture of pharmacologically active flavonolignans which are formed by oxidative coupling of taxifolin (Tx) and coniferyl alcohol (CA). Suspension cultures of this plant constitutively secrete small amounts of Sm into the extracellular medium. Production can be increased by inclusion of cyclodextrins (CDs) in cultures. Both hydroxylated (RHCD) and dimethylated (RMCD) CDs strongly induced prompt accumulation of CA in the medium followed by a late production of flavonolignans. Simultaneous addition of methyl jasmonate (MJ) and RMCD to cells did not significantly modify CA release or flavonolignan accumulation. Delayed addition of MJ to cultures subcultivated in medium containing RMCD markedly influenced Sm production by promoting conversion of the previously formed CA precursor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Silymarin (Sm), a group of flavonolignans of the milk thistle Silybum marianum (L.) Gaernt, is one of the oldest and most widely used traditional European medicine, and has mainly been used for hepatic disorders. Its antioxidative, anti-lipid peroxidative, antifibrotic, anti-inflammatory, membrane stabilizing, immunomodulatory and liver regenerating properties make Sm useful for hepatoprotection in hepatobiliary diseases and in hepatotoxicity due to drugs (Fraschini et al. 2002). New activities based on the specific receptor interactions have been reported, and there is a growing interest in its anticancer and chemopreventive effects which have been demonstrated in a large variety of illnesses of different organs, e.g. prostate, lungs, central nervous system, kidneys, pancreas and also in the skin protection (Kren and Walterova 2005; Gazak et al. 2007).
Sm is composed of an isomeric mixture of the flavonolignans silychristin (Sc), isosilychristin, silydianin (Sd), silybin (Sb) A and B, and isosilybin (ISb) A and B which accumulates in the external cover of seeds (achene fruits) with the precursor flavonoid taxifolin (Tx) and other minor constituents being also present in the Sm complex (Morazzoni and Bombardelli 1995; Kim et al. 2003; Lee and Liu, 2003). The current nomenclature for Sm refers to all these components, although the most active pharmacological compounds are the two pairs of diastereoisomeric flavonolignans Sb A, Sb B, ISb A, and ISb B, which contain 1,4-dioxane ring in addition to flavonoid moiety (Lee and Liu 2003; Shibano et al. 2007).
Most of the publications concerning Sm production by means of cell cultures report a very low content compared with the whole fruit (0.05–0.4% dry weight versus 1–3% in fruits) (Ferreiro et al. 1991; Cacho et al. 1999; Alikaridis et al. 2000; Rahnama et al. 2008). In those studies secondary metabolites were extracted from the biomass and the Sm mixture was composed mainly by Tx and Sc. A more detailed study revealed the presence of Sm in the culture medium of suspensions, with most of individual (Sd, Sb, ISb) components of the Sm group being detected (Sánchez-Sampedro et al. 2005).
Plant cell cultures can be elicited by jasmonates, resulting in increased accumulation of a variety of secondary metabolites (Zhao et al. 2005). Treatment of S. marianum cell cultures with methyl jasmonate (MJ) promoted Sm accumulation both in cells and in the culture medium and, as above, individual components of the Sm mixture were detected in the extracellular medium (Sánchez-Sampedro et al. 2005).
Most approaches to increasing product yields of plant cell cultures have been concentrated on optimization of biosynthesis. Study of other post biosynthetic events, like chemical or enzymatic modifications, transport, storage/secretion and catabolism/degradation are also biotechnologically relevant. Secretion is of particular interest since if cultures are to be used routinely for the commercial production they must release the targeted metabolites into the extracellular medium.
In a recent work, Bru et al. (2006) chose elicitation with cyclodextrins (CDs) as a way to increase production of resveratrol from grapevine cultures and found that application of the chemically pure heptakis(2,6-di-O-methyl)-β-CD caused a dramatic extracellular accumulation of this phytoalexin in the extracellular medium.
CDs are cyclical oligomers of six, seven, or eight glucose molecules derived from starch that can solubilize hydrophobic molecules by virtue of their hydrophobic interior. In plant cells CDs have been employed as precursors solubilizers in biotechnological processes (Van Uden et al. 1994) and as inducers of secondary metabolite production. For example, β-CDs promoted phytoalexin synthesis and extracellular accumulation in grapevine cultures (Bru et al. 2006; Lijavetzky et al. 2008; Morales et al. 1998; Zamboni et al. 2006), induced sesquiterpenes in potato hairy root cultures (Komaraiah et al. 2003) and stimulated menthol production in Mentha cultures (Chakraborty and Chattopadhyay 2008).
Based on this evidence, the main objective of this study has been to explore the ability of CDs to elicit Sm production and secretion in cell cultures of S. marianum. For this, the effect of two modified β-CDs, the randomly methylated (RMCD) and the randomly hydroxypropylated (RHCD) were tested in suspensions. The joint effect of β-CDs and MJ on Sm accumulation was also evaluated.
Materials and methods
Cell cultures
Cell cultures of S. marianum were developed from hypocotyl-derived callus. Suspensions were routinely subcultured every 2 weeks by transferring approximately 10 ml of the previous culture, generated after mixing three parental flasks, to 40 ml Murashige and Skoog medium supplemented with 30 g sucrose l−1, 4.5 μM 2,4-dichlorophenoxyacetic acid and 2.2 μM benzyl adenine at pH 5.6 as reported by Sánchez-Sampedro et al. (2005). Cell cultures were incubated in the dark at 25°C and shaken at 90 rpm.
Chemicals and culture treatments
Hydroxylated (RMCD) dimethylated (HMCD) and cyclodextrins (CD) were purchased from Wacker Chemie (Spain) and Sm, Sb A and Sb B, Tx and CA were from Sigma–Aldrich.
Flasks, 100 ml, containing 20 ml medium were inoculated with cells, harvested by filtration without reduced pressure, from the previous subculture. To the standard growth medium a given concentration of the respective CDs was included before sterilization. MJ (in ethanol) was added to give 100 μM at the times noted in Results. Controls received equivalent volumes of solvent.
Cell viability was checked by differential staining with fluorescein diacetate (Widholm 1972).
Experimental work was performed in triplicate and, at least, in two independent subcultures.
Flavonolignan analysis
Culture medium was separated from the biomass by filtration. Flavonolignans were extracted three times with two volumes of ethyl acetate. The combined extracts were dried in vacuo below 40°C and resuspended in 1 ml methanol. Samples received 0.1 mg naringenin as internal standard before extraction. Analysis was performed by HPLC as described (Sánchez-Sampedro et al. 2005). Identification of CA and flavonolignans was achieved by comparison with commercial standards and by LC MS (MSD trap XCT and LC 1100 both from Agilent), in a Spherisorb S3 ODS2 column (2 × 100 mm, 3.5 μm) in E.S.I (−) under the same conditions as reported for HPLC analysis of flavonolignans.
Chromatograms of the ethyl acetate extracts revealed a prominent peak at 6.1 min. Mass spectra showed the presence in the same peak of a main representative ion [M]− at m/z 178.8 which correspond to CA and one minoritaire at 303.2. Molecular weight of Tx is 304.3 therefore the ion at 303.2 probably represented this flavonoid. However, due to its limited presence, its levels could not be quantified throughout this work and in the results, absolute values were given only for CA. Peaks at retention times other than 6.1 showing a molecular ion [M]− at m/z 481.2 corresponded to Sm.
Results and discussion
During the normal culture cycle of S. marianum suspensions, Sd and traces of Sb and ISb could be measured in the spent medium (Fig. 2a). The ability of β-CDs to improve the production of these flavonolignans is shown in Fig. 2b and c. Compared with control, addition of RHCD and RMCD intensely stimulated CA release in the culture medium. CA appreciably accumulated after 24 h of CD treatment and steadily increased up to 72–96 h; in contrast, compounds of the Sm group increased gradually with time up to the 7 days concomitantly with a decrease in CA, which, as already mentioned, is the precursor phenylpropanoid of the flavonolignan isomeric mixture. Cell growth under the experimental conditions employed in this work is shown in Fig. 1.
Growth (measured as dry weight) of cell suspension of S. marianum in the presence of 50 mM RHCD (–∆–), 50 mM RMCD (–○–), 100 μM MJ (–■–) or 50 mM RMCD + 100 μM MJ (–●–) during a cultivation period of 7 days. (–▲–) Growth of cultures which received 100 μM MJ after 3 days in contact with 50 mM RMCD. (–□–) Control. The values represent the average of three replicate experiments ± SD
Effect of β-CDs on CA and Sm production in cell cultures of S. marianum. a Control cultures. b Suspensions cultured in the presence of 50 mM RHCD. c Suspensions cultured in the presence of 50 mM RMCD. (□) CA, ( ) Sd, (■) Sb, (
) Sd, (■) Sb, ( ) ISb. Experiments were repeated at least twice with similar results. Data represent mean of triplicate cultures ± SD
) ISb. Experiments were repeated at least twice with similar results. Data represent mean of triplicate cultures ± SD
By forming adducts (stable inclusion complexes) with β-CDs, the solubility of molecules can increase considerably. In fact, it has been shown that the bioconversion of CA into podophyllotoxin in Podophyllum hexandrum cell cultures was successfully enhanced by feeding 3 mM CA (whose solubility in water is up to 0.15 mM) solubilised with a β-CDs (Van Uden et al. 1994). Empty CDs molecules, besides stimulating CA production, could thus also serve as adsorbents for CA. This latter function not only could lead to the substantial accumulation of this compound but also may prevent the possible toxic and/or inhibitory effects of CA on the S. marianum cells.
The effectiveness of CDs was dependent on both concentration and exposure time. Figure 3 shows the effect of different concentrations on CA release and Sm accumulation at day 3 and 6 after treatment. Although 100 mM caused the highest CA release, this concentration was not suitable for latter flavonolignan accumulation (see data at day 6), since it severely reduced growth and compromised cell viability after prolonged treatment (data not shown). RMCD was more effective in stimulating Sm accumulation than RHCD; therefore, further experiments were performed with the randomly methylated form.
Effect of different concentrations of RHCD (a) or RMCD (b) on CA and Sm production in S. marianum cultures. Results were evaluated 3 or 6 days after treatment. (□) CA, ( ) Sd, (■) Sb, (
) Sd, (■) Sb, ( ) ISb. Experiments were repeated at least twice with similar results. Data represent mean of triplicate cultures ± SD
) ISb. Experiments were repeated at least twice with similar results. Data represent mean of triplicate cultures ± SD
MJ elicits Sm production in S. marianum (Sánchez-Sampedro et al. 2005). In order to test whether addition of MJ improved the RMCD responses, 100 μM MJ was added to RMCD-treated suspensions on day 0 or day 3 of the culture cycle and CA and flavonolignans were then evaluated. As presented in Fig. 4, MJ added alone at day 0 stimulated CA release although to a much lesser extent than RMCD; MJ effects on Sm compounds were only seen after 3–4 days after treatment and experiments could not proceed beyond 6 days due to loss of cell viability (data not shown). Neither CA release nor Sm accumulation was modified when cells were simultaneously treated with MJ and RMDC (see Fig. 4).
Effect of joint addition of MJ and RMCD on CA and Sm production in S. marianum cultures. Cell suspensions were treated with 100 μM MJ + 50 mM RMCD at day 0 after subculture. Products were extracted from the culture medium at different incubation periods. (□) CA, ( ) Sd, (■) Sb, (
) Sd, (■) Sb, ( ) ISb. Experiments were repeated at least twice with similar results. Data represent mean of triplicate cultures ± SD
) ISb. Experiments were repeated at least twice with similar results. Data represent mean of triplicate cultures ± SD
Addition of MJ to cultures that had been treated with RMCD for 3 days enhanced Sm accumulation. As shown in Fig. 5, accumulation started 24 h after MJ treatment and, at 72 h (6 days after subculture), total Sm reached 1.5 ± 0.1 mg per flask, 5.2, 3.67 and 1.7 times greater than that of control, MJ- or RMCD-treated cultures respectively. This strategy reduced the amount of the precursor CA in the medium (see Fig. 5) and rendered, up to date, the highest values of the more active components of the Sm mixture seen in cell cultures of this plant species (near 0.5 mg Sb, and 0.18 mg ISb per flask). The results also suggest that MJ, besides stimulating precursor release into the medium, promoted conversion of CA into flavonolignans.
Effect of delayed addition of MJ on CA and Sm production in S. marianum cultures pretreated with 50 mM RMCD for 3 days. Products were extracted from the culture medium after incubation periods of 1, 2 and 3 days in the presence of 100 μM MJ. (□) CA, ( ) Sd, (■) Sb, (
) Sd, (■) Sb, ( ) ISb. Experiments were repeated at least twice with similar results. Data represent mean of triplicate cultures ± SD
) ISb. Experiments were repeated at least twice with similar results. Data represent mean of triplicate cultures ± SD
Zamboni et al. (2009) showed that in grapevine cultures 50 mM RMCD initiated a signal transduction cascade leading to the activation of stilbene and monolignol biosynthesis. The authors also observed the induction of genes encoding putative secondary metabolite transporters, such as those belonging to the ATP-binding cassette (ABC) transporter family, thus explaining the accumulaton of stilbenes in the growth medium. While in Silybum control cultures CA release into the culture medium can be the consequence of the stress imposed by subcultivation, the RMCD-induced CA release from cells into the medium could be regarded as a combination of both induced synthesis and excretion via activation of specific transport systems. This hypothesis should be considered in future work.
Conclusion
We report the optimized production and secretion of CA and flavonolignan compounds in S. marianum cell cultures. The production of these secondary metabolites in cultures can be maximized by employing CDs as inducers of CA release into the extracellular medium and its conversion into active flavonolignans by a delayed addition of MJ.
References
Alikaridis F, Papadakis D, Pantelia K, Kephalas T (2000) Flavonolignan production from Silybum marianum transformed and untransformed root cultures. Fitoterapia 71:379–384
Bru R, Selles S, Casado-Vela J, Casado-Vela S, Pedreño MA (2006) Modified cyclodextrins are chemically defined glucan inducers of defense responses in grapevine cell cultures. J Agric Food Chem 54:65–71
Cacho M, Morán M, Corchete P, Fernández-Tárrago J (1999) Influence of medium composition on the accumulation of flavonolignans in cultured cells of Silybum marianum (L.) Gaertn. Plant Sci 144:63–68
Chakraborty A, Chattopadhyay S (2008) Stimulation of menthol production in Mentha piperita cell culture. In Vitro Cell Dev Biol Plant 44:518–524
Ferreiro P, Pais MSS, Cabral JMS (1991) Production of silybin-like compounds in cell suspension cultures of Silybum marianum. Planta Med 57:2–3
Fraschini F, Demartini G, Esposti D (2002) Pharmacology of silymarin. Clin Drug Invest 22:51–65
Gazak R, Walterova D, Kren V (2007) Silybin and silymarin: new and emerging applications in medicine. Curr Med Chem 14:315–338
Kim NC, Graf TN, Sparacino CM, Wani MC, Wall ME (2003) Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum). Org Biomol Chem 1:1684–1689
Komaraiah P, Reddy GV, Srinivas Reddy P, Raghavendra AS, Ramakrishna SV, Reddanna P (2003) Enhanced production of antimicrobial sesquiterpenes and lipoxygenase metabolites in elicitor-treated hairy root cultures of Solanum tuberosum. Biotechnol Lett 25:593–597
Kren V, Walterova D (2005) Silybin and silymarin new effects and applications. Biomed Papers 149:29–41
Lee DYW, Liu YZ (2003) Molecular structure and stereochemistry of silybin A, silybin B, isosilybin A, and isosilybin B, isolated from Silybum marianum (milk thistle). J Nat Prod 66:1171–1174
Lijavetzky D, Almagro L, Belchi-Navarro S, Martínez-Zapater JM, Bru R, Pedreño MA (2008) Synergistic effect of methyl jasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Res Notes 1:132
Morales M, Bru R, García-Carmona F, Ros-Barceló A, Pedreño MA (1998) Effect of dimethyl-β-cyclodextrins on resveratrol metabolism in Gamay grapevine cell cultures before and after inoculation with shape Xylophilus ampelinus. Plant Cell Tissue Organ Cult 53:179–187
Morazzoni P, Bombardelli E (1995) Silybum marianum (Carduus marianum). Fitoterapia 66:3–42
Ohvo H, Slotte JP (1996) Cyclodextrin-mediated removal of sterols from monolayers: effects of sterol structure and phospholipids on desorption rate. Biochemistry 35:8018–8024
Rahnama H, Hasanloo T, Shams MR, Sepehrifar R (2008) Silymarin production by hairy root culture of Silybum marianum (L.) Gaertn. Iranian J Biotechnol 6:113–118
Sánchez-Sampedro MA, Fernández-Tárrago J, Corchete P (2005) Yeast extract and methyl jasmonate-induced silymarin production in cell cultures of Silybum marianum (L.) Gaernt. J Biotechnol 119:60–69
Shibano M, Lin AS, Itokawa H, Lee KH (2007) Separation and characterization of active flavonolignans of Silybum marianum by Liquid Chromatography connected with Hybrid Ion-Trap and Time-of-Flight Mass Spectrometry (LC–MS/IT-TOF). J Nat Prod 70:1424–1428
Van Uden W, Woerdenbag HJ, Pras N (1994) Cyclodextrins as a useful tool for bioconversions in plant cell biotechnology. Plant Cell Tiss Org Cul 38:103–113
Widholm JM (1972) The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol 47:189–194
Zamboni A, Vrhovsek U, Kassemeyer HH, Mattivi F, Velasco R (2006) Elicitor-induced resveratrol production in cell cultures of different grape genotypes (Vitis spp.). Vitis 45:63–68
Zamboni A, Gatto P, Cestaro A, Pilati S, Viola R, Mattivi F, Moser C, Velasco R (2009) Grapevine cell early activation of specific responses to DIMEB, a resveratrol elicitor. BMC Genomics 10:363–373
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333
Acknowledgments
This work was financed by Ministerio de Ciencia e Innovación (BFU2008-02876/BFI), and by the Consejería de Educación, Ciencia e Investigación de la Región de Murcia (BIO BVA 07 01 0003), Spain. We are grateful to Dr. C. Raposo, from the Spectroscopy Service of the University of Salamanca, for his valuable help at identifying CA and Sm compounds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Belchi-Navarro, S., Pedreño, M.A. & Corchete, P. Methyl jasmonate increases silymarin production in Silybum marianum (L.) Gaernt cell cultures treated with β-cyclodextrins. Biotechnol Lett 33, 179–184 (2011). https://doi.org/10.1007/s10529-010-0406-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-010-0406-6