Abstract
A triple-point mutated fish microsomal epoxide hydrolase (mEH) gene from Mugil cephalus was expressed in Escherichia coli in the presence of various chaperones to prevent protein aggregations. The enantioselective hydrolytic activity was more than doubled by co-expressing the EH mutant gene with pGro7 plasmid. The highly active EH mutant with a his-tag was immobilized onto magnetic silica assembled with NiO nanoparticles. The immobilized mEH mutant was re-used more than 10 times with less than 10% activity loss. (S)-Styrene oxide with 98% enantiopurity was repeatedly obtained with over 50% of the theoretical yield by the magnetically separable high-performance mEH mutant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chiral epoxides are enantiopure synthetic intermediates with excellent reactivity (de Vries and Janssen 2003; Hwang et al. 2010; Kasai et al. 1998). They can be prepared by resolution of racemic epoxides by using epoxide hydrolase (EH) (Steinreiber and Faber 2001; Widersten et al. 2010). Recently, a fish microsomal EH (mEH) gene from Mugil cephalus has been cloned and characterized (Lee et al. 2007). The catalytic activity of M. cephalus EH has been enhanced 35-fold by site-directed mutagenesis based on comparative modeling (Choi et al. 2009). The triple-point mutant, F193Y for spatial orientation of the nucleophile (D199), W200L for removing electron density overlap between W200 and Y348, and E378D for good charge relay in the active site, was developed.
In order to enhance the economic feasibility of kinetic resolutions, biocatalysts need to be recycled efficiently (Brady and Jordaan 2009). Nanostructured materials have low mass transfer limitation due to their reduced thickness of particles, and can offer high enzyme loading due to large surface area (Kim et al. 2006). In the case of magnetic nanoparticle-immobilized enzymes, they can be readily separated by applying an external magnet, which allow efficient recycling of the immobilized enzymes. Recently, a novel magnetic nanoparticles presenting NiO on the surface was developed for the separation of histidine-tagged (His-tagged) recombinant proteins (Lee and Lee 2008, Lee et al. 2009).
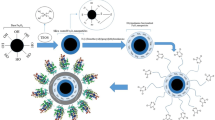
High-level expression of enzymes in Escherichia coli is widely used Sørensen and Mortensen 2005). The heterologous expression of eukaryotic microsomal genes in E. coli as an active form, however, is often difficult due to the formation of insoluble inclusion bodies. Chaperones can be employed to prevent misfolding and aggregation, thus help heterologous proteins to be expressed in more active form (Fink 1999). In this paper, a triple-point mutated gene of M. cephalus mEH was functionally expressed by co-expressing chaperones. The highly active EH mutant was separated and immobilized onto the surface of magnetic silica displaying NiO nanoparticles (Fig. 1). The immobilized EH biocatalysts were applied for preparing (S)-styrene oxide in a repeated batch mode.
Immobilization of triple-point EH mutant on magnetic nanoparticle based on the affinity between his-tag and hybrid Fe3O4/silica/NiO nanoparticles, and its application to repetitive enantioselective resolution of racemic styrene oxide. PEG-HNP and HIS-HNP represent the hybrid Fe3O4/silica/NiO nanoparticle and his-tagged enzyme-immobilized hybrid nanoparticle, respectively. ((R)-SO (R)-styrene oxide, (S)-SO (S)-styrene oxide)
Materials and methods
Strains and culture conditions
The recombinant plasmid containing the triple-point mutated gene of M. cephalus mEH (pET-21b(+)/mMcEH triple mutant) was used in this study (Choi et al. 2009). To analyze the effect of the co-expression of the mutated EH gene with different combinations of chaperones, the recombinant plasmid was transformed into various E. coli BL21 (DE3) harboring chaperone plasmids (pG-KJE8, pGro7, pKJE7, pG-Tf2 and pTf16) (Takara, Japan). The resulting E. coli BL21 (DE3) was cultured on LB containing 50 μg ampicillin/ml and 34 μg chloramphenicol/ml at 37°C for 2 h with shaking at 190 rpm. l-Arabinose and tetracycline were added to 0.5 mg/ml and 5 ng/ml, respectively, for the induction of chaperone expression. When OD600 of the culture reached 0.4–0.6, the cells were incubated at 15°C for 24 h to express the mutated EH gene by the addition of 1 mM IPTG.
Purification of the mutated EH proteins
Cells were harvested by centrifugation and re-suspended in 50 mM sodium phosphate lysis buffer containing 300 mM NaCl, 10 mM imidazole, 10% (v/v) glycerol and 0.5% (v/v) Triton X-100. DNase, lysozyme and PMSF were added to 0.02 mg/ml, 1 mg/ml and 1 mM, respectively. The suspension solution was ultrasonicated for 20 min with ice cooling. The cell debris was precipitated by centrifugation (10000×g, 20 min) at 4°C, and the supernatant was filtered through a 0.2 μm membrane to obtain a clear extract. The resulting supernatant containing His-tagged EH mutant and chaperones was loaded on Ni-Sepharose column equilibrated with 20 mM phosphate buffer (pH 7.4), 0.5 M NaCl and 20 mM imidazole. The column was washed with same buffer containing 100 mM imidazole for 1 h at 1 ml/min to remove chaperones. Finally, His-tagged EH mutant was eluted with same buffer containing 400 mM imidazole. The active fraction was desalted using HiTrap desalting column. The proteins were analyzed by 12% (v/v) SDS-PAGE as described previously (Choi et al. 2009) with BSA (66 kDa), albumin from chicken egg white (45 kDa) and carbonic anhydrase (29 kDa) was used as a protein marker.
To analyze the proteins of the periplasmic space, cells from 150 ml culture were harvested by centrifugation and the pellet was resuspended in 1.25 ml of 30 mM Tris/HCl containing 25% (w/v) sucrose (pH 8.0). Then, 50 μl of 0.25 M EDTA, 50 μl lysozyme (20 mg/ml in water), and 1.25 ml distilled water were added sequentially. The samples were centrifuged at 5,000×g for 10 min to separate the periplasm from the spheroplasts (Tran et al. 2005).
Enantioselective resolution of racemic styrene oxide
Enantioselective resolution of racemic styrene oxide was conducted in 1 ml 100 mM potassium phosphate buffer (pH 7.4) in 10 ml screw-cap bottles sealed with a rubber septum. Enantioselective resolution reaction was started with the addition of 20 mM racemic styrene oxide at 30°C and shaking at 230 rpm. The reaction was stopped by extraction with equal volume of cyclohexane. The organic phase was analyzed for the determination of enantiopurity and yield by GC analysis as described previously (Choi et al. 2009). To analyze the inhibitory effects of the diol product, various concentrations of enantiopure and racemic phenyl-1,2-ethanediol were added to reaction mixtures and the initial hydrolysis rates were measured.
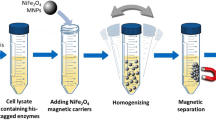
Immobilization of the EH mutant on the hybrid Fe3O4–silica–NiO nanoparticles and re-use of the immobilized EH mutant in a repeated batch operation
PEG-modified hybrid Fe3O4–silica–NiO nanoparticles (PEG-HNPs) were prepared (see Lee et al. 2009). EH mutant, 200 μg/ml, with the his-tag was immobilized onto 10.8 mg magnetic nanoparticles displaying NiO on their surface in 100 mM potassium phosphate buffer (pH 7.4) at 25°C for 30 min. After the enantioselective resolution reaction was carried out by adding 20 mM racemic styrene oxide into the phosphate buffer (pH 7.4) containing the immobilized EH mutant with steady stirring at 30°C, the biocatalyst was isolated by applying magnetic bar. New reaction buffer with the given amounts of substrate was added to the immobilized biocatalyst for further enantioselective resolutions.
Analysis
Enantiomeric excess (ee = 100 × (S − R)/(S + R)) and yield for enantiopure styrene oxide were determined by GC analysis as described previously (Choi et al. 2009).
Results and discussion
Functional expression of the triple-point mutated fish microsomal EH gene in E. coli via chaperone co-expression
Recently, we have cloned and engineered the microsomal EH of M. cephalus to enhance its activity more than 35-fold (Choi et al. 2009). However, overproduction of the EH mutant in E. coli was not reliable. Large quantities of insoluble protein aggregates were formed because the microsomal EH gene was originally cloned from a marine fish. Chaperone-assisted expression was considered to produce the active EH mutant in soluble form. The triple-point mutated EH gene was expressed in the presence of various chaperone plasmids, and the expression profiling are shown in Fig. 2. The EH mutant gene was successfully expressed in the presence of various chaperones, and the expression level of the EH mutant was slightly low in the presence of chaperones compared to the EH mutant alone.
SDS-PAGE analysis of the triple-point mutated M. cephalus EH in E. coli BL21 with chaperone plasmid. M indicates marker. (a), (b), (c), (d), (e) and (f) represent BL21, BL21/pG-KJE8, BL21/pG-Tf2, BL21/pGro7, BL21/pTf16, and BL21/pKJE7, respectively. Lane 1: whole cell, Lane 2: lysate, and Lane 3: periplasmic space
The enantioselective hydrolytic activities of the whole cells of recombinant E. coli expressing the triple-point mutated EH gene in the presence of various chaperones were compared. Among the tested six recombinants, the recombinant E. coli with pGro7 exhibited the highest activity. The pGro7 contains the genes for groES–groEL that play a key role in protein folding after translation in prokaryotic cells. When the enantioselective hydrolysis of 20 mM racemic styrene oxide was conducted, the reaction time to reach 98%ee decreased from 24 to 13 min for the recombinant E. coli with co-expression of groES–groEL, compared to the recombinant E. coli without any chaperones (Fig. 3a, b). The average initial hydrolysis rates were 0.28 and 0.57 mmol/min mg for the whole cells in the absence and presence of groES–groEL, respectively. Even though the expression level of the triple-point mutated EH was low in the presence of pGro7 than that in the absence of any chaperone, the activity was approximately two-fold enhanced on the basis of the same amount of dry cell weight.
Batch enantioselective resolution of racemic styrene oxides by the recombinant whole cells expressing the M. cephalus EH mutant without any chaperones (a) and in the presence of pGro7 plasmid (b), and by the isolated EH mutant protein expressed in the presence of pGro7 plasmid (c). Open circle (S)-styrene oxide, filled circle (R)-styrene oxide
Characterization of the triple-point mutated EH protein co-expressed with groES–groEL
To analyze the specific activity of the triple-point mutated EH expressed in the presence of groES–groEL, the EH mutant proteins were separated with Ni-Sepharose columns. The enantioselective resolution of racemic styrene oxides was conducted by using the EH mutant proteins (Fig. 3c). The specific hydrolysis rate to racemic styrene oxide of the triple-point mutated EH expressed in the presence of groES–groEL was about 1.4 mM mmol/min mg, whereas that in the absence of chaperones was 1 mM mmol/min mg. The hydrolytic activity of the triple-point mutated EH expressed in the presence of groES–groEL was somewhat low than expected. This is because some portion of the EH mutant protein was denatured probably due to the removal of chaperones during the purification step. In order to confirm the positive effect of chaperones in vitro, the isolated chaperones during EH purification were added to 2 ml 100 mM phosphate buffer (pH 7.4) containing 150 μg/ml of the EH mutant some portion of which has been aggregated during storage after purification. When the amount of chaperone groEL–groES increased from 0 to 280 μg/ml, the hydrolysis rate for styrene oxide increased from 0.16 mM to 0.45 mM mmol/min mg, which indicates that chaperones assisted the renaturation of the mEH mutant in vitro.
The effects of pH and temperature on the relative activity of the EH mutant proteins were analyzed (Fig. 4a, b). The optimal pH and temperature were 8 and 40°C, respectively. The EH mutant exhibited a relative activity more than 80% from pH 7 to 9 and from 15 to 30°C.
Effects of pH and temperature on the activity of the EH mutant. a and b for the isolated EH mutant expressed in the presence of groEL-groES. c and d for the nano-immobilized EH biocatalyst. Filled circle 100 mM sodium acetate/acetic acid buffer, open circle 100 mM potassium phosphate, Filled inverted triangle 100 mM Tris/HCl, open triangle 100 mM glycine/NaOH. For determination of the optimal reaction temperature, EH activities were measured at pH 7.4 over the different temperatures ranging from 10 to 50°C as described previously (Lee et al. 2007)
The kinetic properties of the isolated triple-point mutated EH that was expressed in the presence of groES–groEL were analyzed. To prevent experimental errors due to the mass-transfer limitation of the substrate caused by its poor solubility, we conducted kinetic analysis experiments within the range of the substrate’s solubility. Initial hydrolysis rates were determined at various concentrations of (S) and (R)-styrene oxide from 0 to 6 mM. The hydrolysis rates of (S) and (R)-styrene oxide followed the Michaelis–Menten kinetic model. The maximum hydrolysis rate (V max) and Michaelis–Menten constant (K m) for both enantiomers were determined by double-reciprocal plots. V Smax and K Sm for (S)-styrene oxide were 5.5 mmol/min mg and 5.6 mM, respectively, while V Rmax and K R m for (R)-styrene oxide were 9.9 mmol/min mg and 2 mM, respectively. In the case of the wild-type M. cephalus EH, it has a V Smax value of 0.3 mmol/min mg, a K Sm value of 4 mM, a V Rmax value of 0.32 mmol/min mg and a K Rm value of 3.4 mM. The enantiomeric ratio, E = (V R max /K Rm )/(V Smax /K Sm ), of the triple-point mutated EH protein were around 5.1, which is similar to that of the wild-type mEH protein. The maximum degradation rates for both (S)- and (R)-styrene oxide of the EH mutant in the presence of groES–groEL were more than 18- and 31-fold higher than those of the wild-type mEH, respectively.
The effect of phenyl-1,2-ethanediol, the hydrolysis product of styrene oxide, on the activity of the EH mutant was investigated. The initial hydrolysis rates were measured in the presence of various amounts of (R)-phenyl-1,2-ethanediol, (S)-phenyl-1,2-ethanediol and racemic phenyl-1,2-ethanediol. Up to 200 mM, there was no or little inhibition by the diols to the EH mutant expressed in the presence of groES–groEL, which is an advantageous property in enantioselective resolutions at high substrate concentration (data not shown).
Immobilization of the triple-point mutated EH enzyme onto magnetic silica nanoparticles
The hybrid nanoparticles of Fe3O4/silica/NiO modified with PEG (PEG-HNPs) were prepared for the immobilization of the His-tagged EH mutant. The amount of the EH mutant immobilized onto 1 mg PEG-HNPs was approximately determined to be 18.5 μg. Based on the same amount of enzyme, more than 90% of enzyme activity was maintained after immobilization. In order to determine the optimal environmental conditions for the immobilized EH, the effect of pH and temperature were investigated (Fig. 4c, d). The optimal pH and temperature were 8.0 and 40°C, respectively, which are similar to those of free enzymes. The activity of the immobilized EH, however, was low at high temperature above 40°C and very low at low pH below 6. The immobilized EH enzyme maintained a catalytic activity for narrow range of pH and temperature, compared to the free EH. The immobilized EH had the same enantiopreference that (R)-styrene oxide was preferentially hydrolyzed. These data suggests that the immobilization did not significantly affect the catalytic properties of the enzyme.
The repetitive preparation of enantiopure (S)-styrene oxide was conducted by consecutive additions of 20 mM racemic styrene oxide. As shown in Table 1, the immobilized EHs were successfully re-used for more than 10 times without significant loss of the residual activity. In the first cycle, (S)-styrene oxide was obtained within 20 min with 28.9% yield. The immobilized EH biocatalysts were recovered by magnetic attraction, and then efficiently re-used in consecutive reactions. In the following cycles, the reaction time required to reach 98% enantiopurity was slightly increased. When the initial hydrolytic rates were measured and compared, the loss of activity at each cycle was below 10%. Hence, reuse of the immobilized EH enzymes onto the magnetic nanoparticles proved to be an efficient method for the repetitive preparation of (S)-styrene oxide with more than 98%ee.
Conclusions
The triple-point mutated fish mEH gene was functionally expressed in E. coli BL21 (DE3) with co-expression of pGro7 plasmid harboring groES–groEL genes. The EH mutant expressed in the presence of groES–groEL exhibited a two-fold enhanced enantioselective hydrolysis activity. The EH mutant with his-tag could be readily immobilized onto the hybrid nanoparticles of Fe3O4/silica/NiO based on the affinity between his-tag and NiO nanoparticles. The immobilization procedure was very simple, and the immobilized EH biocatalyst was efficiently recovered in the repeated batch operations. (S)-styrene oxide with an enantiopurity of 98%ee could be obtained by using the immobilized EH, and the activity and stability of the immobilized EH were successfully maintained for more than 10 cycles.
References
Brady D, Jordaan J (2009) Advances in enzyme immobilization. Biotechnol Lett 31:1639–1650
Choi SH, Kim HS, Lee EY (2009) Comparative homology modeling-inspired protein engineering for improvement of catalytic activity of Mugil cephalus epoxide hydrolase. Biotechnol Lett 31:1617–1624
de Vries EJ, Janssen DB (2003) Biocatalytic conversion of epoxides. Curr Opin Biotechnol 14:414–420
Fink AL (1999) Chaperone-mediated protein folding. Physiol Rev 79:425–449
Hwang S, Choi CY, Lee EY (2010) Bio- and chemo-catalytic preparation of chiral epoxides. J Ind Eng Chem 16:1–6
Kasai N, Suzuki T, Furukawa Y (1998) Chiral C3 epoxides and halohydrins: Their preparation and synthetic application. J Mol Catal B: Enzym 4:237–252
Kim J, Grate JW, Wang P (2006) Nanostructures for enzyme stabilization. Chem Eng Sci 61:1017–1026
Lee KS, Lee IS (2008) Decoration of superparamagnetic iron oxide nanoparticles with Ni2+: agent to bind and separate histidine-tagged proteins. Chem Comm 2008:709–711
Lee SJ, Kim HS, Kim SJ, Park S, Kim BJ, Shuler ML, Lee EY (2007) Cloning, expression and enantioselective hydrolytic catalysis of a microsomal epoxide hydrolase from a marine fish, Mugil cephalus. Biotechnol Lett 29:237–246
Lee KS, Woo MH, Kim HS, Lee EY, Lee IS (2009) Synthesis of hybrid Fe3O4/silica/NiO superstructures and their application as magnetically separable high-performance biocatalysts. Chem Comm 25:3780–3782
Sørensen HP, Mortensen KK (2005) Advanced genetic strategies for recombinant protein expression in Escherichia coli. J Biotechnol 115:113–128
Steinreiber A, Faber K (2001) Microbial epoxide hydrolases for preparative biotransformations. Curr Opin Biotechnol 12:552–558
Tran TAT, Struck DK, Young R (2005) Periplasmic domains define holin-antiholin interactions in T4 lysis inhibition. J Bacteriol 187:6631–6640
Widersten M, Gurell A, Lindberg D (2010) Structure-function relationships of epoxide hydrolases and their potential use in biocatalysis. Biochim Biophys Acta 1800:316–326
Acknowledgment
This work was supported by the Marine and Extreme Genome Research Center Program, Ministry of Land, Transportation and Martime Affairs, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, S.H., Kim, H.S., Lee, I.S. et al. Functional expression and magnetic nanoparticle-based Immobilization of a protein-engineered marine fish epoxide hydrolase of Mugil cephalus for enantioselective hydrolysis of racemic styrene oxide. Biotechnol Lett 32, 1685–1691 (2010). https://doi.org/10.1007/s10529-010-0335-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-010-0335-4