Abstract
We compared the effect of the rice storage protein glutelin B-1 (GluB-1) terminator with the nopaline synthase (Nos) terminator on the accumulation of the modified house dust mite allergen mDer f 2 driven by the maize ubiquitin promoter in transgenic rice. Accumulation of mDer f 2 in transgenic seed and leaf using the GluB-1 terminator was greater than when using the Nos terminator construct. The mDer f 2 mRNA containing the GluB-1 3′UTR was processed and polyadenylated at the same sites as the native GluB-1 mRNA in the seeds but diverged in leaves of the transgenic plants. In contrast, the poly(A) sites of mDer f 2 containing Nos 3′UTR were more divergent in both seed and leaf. These results suggest that GluB-1 3′UTR functions as a faithful terminator and that termination at the specific sites may play an important role in mRNA stability and/or translatability, resulting in higher levels of protein accumulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transgenic plants are a promising platform for the production of valuable protein. Yields of recombinant products are therefore critical factors for the practical application of plant-based production. High levels of accumulation of recombinant proteins can be achieved using strong specific promoters, stabilizing mRNA, controlling translation of protein, choosing suitable subcellular localization (compartment) and further selecting appropriate 3′-untranslated regions (3′UTRs) as terminators (Streatfield 2007; Knirsch et al. 2000; Ali and Taylor 2001).
De Jaeger et al. (2003) reported that use of common bean seed storage protein arcelin 5-1 and β-phaseolin promoters together with the arcelin 5-1 terminator enhanced the accumulation of functional murine scFv to 13% and 37%, respectively, of total soluble proteins in transgenic dicotyledonous seeds. Takaiwa et al. (2008) have achieved high level accumulation of potentially clinically exploitable peptides in transgenic rice seeds using the rice storage protein gene glutelin B-1 (GluB-1) promoter and its terminator, in some cases achieving a yield of approximately 15% of total seed proteins. However, the nopaline synthase (Nos) terminator combined with heterologous promoters is more often used for the expression of transgenes in plants (Depicker et al. 1982). In general, the levels of expression of reporters such as GFP or GUS genes are satisfactory for studies on biochemistry, physiology and cellular localization of plants even when the Nos 3′-terminator is used. However, for optimal accumulation of recombinant proteins in practical applications and large-scale production, the functions of 3′UTRs need to be studied further in transgenic plants.
The 3′UTRs of several plant genes have been found to contribute to quantitative regulation of gene expression. The 3′UTR of the C4 photosynthesis gene Mel increased expression of the GUS reporter gene in leaves of transgenic plants without altering the pattern of expression (Ali and Taylor 2001). The 3′UTR of the manganese superoxide dismutase gene functions as a translational enhancer in vivo (Knirsch and Clerch 2000), while the 3′UTR of soybean cytosolic glutamine synthetase gene plays a major role in both transcript turnover and translation repression in transgenic alfalfa (Ortega et al. 2006).
Here, the function of the 3′UTRs from GluB-1 and Nos as terminators was examined by expressing the modified major house dust mite allergen mDer f 2 gene in transgenic rice under the control of the maize ubiquitin constitutive promoter. mDer f 2 modified by altering the tertiary structure by deleting disulphide bonds is expected to be a safer tolerogen for allergen-specific immunotherapy, because it cannot be recognized by specific IgE antibodies (Takai et al. 1997). mDer f 2 transcript and protein levels in transgenic rice seeds were about 1.8- and 4-fold higher, respectively, using the GluB-1 terminator than with the Nos terminator construct. These results indicate that the GluB-1 3′UTR was responsible for both transcriptional and post-transcriptional control of mDer f 2 accumulation.
Materials and methods
Plant transformation
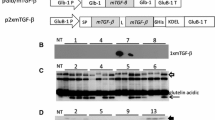
The Der f 2 gene (accession number CQ768107) was optimized for translation based on codons frequently used in rice seed storage protein genes, and was synthesized by the GenScript Corporation (CA, USA). To change the tertiary structure from that required for specific IgE binding, cysteine residues at positions 8, 21, 27, 73, 78 and 119 of the mDer f 2 sequence involved in disulphide bond formation were replaced by serine residues and the modified gene was ligated downstream of the maize ubiquitin promoter (Christensen and Quail 1996). A GluB-1 signal peptide sequence and a KDEL ER retention signal were attached to the N- and C-termini of the gene, respectively, followed by the 0.62 kb GluB-1 or the 0.26 kb Nos terminators (Fig. 1). The gene cassettes were then cloned into the HindIII and EcoRI sites of binary vector pGPTV-35S-HPT (Goto et al. 1999) and the resulting plasmids were designated pUbiDerGluB and pUbiDerNos, respectively.
Diagram of pUbiDerGluB and pUbiDerNos vectors used for transformation of rice. Hpt hygromycin phosphotransferase gene; pAg7 3′-terminal region of the agropine synthase gene; 35S P CaMV 35S promoter; Ubi-P, GluB-1 SP, GluB1 T and Nos T represent maize ubiquitin promoter, glutelin B1 signal peptide sequence, glutelin B1 and nopaline synthase gene terminators, respectively; mDer f 2-KDEL, modified Der f 2 containing a KDEL ER retention signal at its C-terminus; RB and LB are T-DNA right and left borders
The expression plasmids were introduced into rice (Oryza sativa L. cv. Kita-ake) by Agrobacterium tumefaciens-mediated transformation, as described previously, and the resulting transgenic plants were grown in a controlled greenhouse at 28°C with 12 h light/dark cycles (Goto et al. 1999).
Antibody preparation
The DNA sequence encoding mature mDer f 2 protein was cloned into the expression plasmid pET23d(+) at the NcoI and SacI sites, and transformed into E. coli BL21 (DE3) (Novagen, Tokyo, Japan). Expressed mDer f 2 was purified with the QIAexpressionistTM kit according to the manufacturer’s instructions (Qiagen, Tokyo, Japan) and used to raise rabbit anti-mDer f 2 antibody (Qiagen, Tokyo, Japan).
Confirmation of transgenic plants
Rice seed, leaf and root were ground to a fine powder with a Multibeads shocker (Yasui Kikai, Osaka, Japan), and total proteins were extracted in Urea–SDS buffer [50 mM Tris/HCl, pH 6.8, 8 M urea, 4% (v/v) SDS, 5% (v/v) 2-mercaptoethanol, 20% (v/v) glycerol]. After separation by 12% SDS-PAGE and transfer to PVDF membrane (Millipore, MA, USA), mDer f 2 protein was detected with anti-mDer f 2 antibody followed by a goat anti-rabbit IgG secondary antibody conjugated to horseradish peroxidase (Cell Signaling, MA, USA). For determination of the accumulation of mDer f 2 in seeds, at least four positive seeds from each line were mixed and subjected to quantitative immuno-dot-blots with anti-mDer f 2 antibody. The dots were quantified with NIH image J software (National Institutes of Health, Washington DC, USA).
RT-PCR and real-time RT PCR
Total RNAs were purified from 10-day-old leaf and 15-day-old developing seed of T3 homozygous transgenic rice plants as previously described (Takaiwa et al. 1987). Aliquots of 1 μg of total RNA were subjected to first-strand cDNA synthesis using the Superscript III First-Strand Synthesis System for Quantitative Real-Time PCR (Invitrogen, CA, USA) according to the manufacturer’s protocol. The mDer f 2 expression levels were measured by RT-PCR and real-time RT PCR using specific primers mDer-f2-FD (5′-CAAAACACCAAGACCGCAAA-3′) and mDer-f2-RV (5′-GTTGGCCCTTAACGAGTGGA-3′) together with the synthesized cDNAs as templates; the length of amplified PCR product is about 150 bp. The 17S rRNA was determined in each sample for normalization of starting RNA concentrations in RT-PCR analysis and the primers for 17S rRNA amplification were 17S rRNA-FD (5′-ACACGGGGAAACTTACCAGGTC-3′) and 17S rRNA-RV (5′-CCAGAACATCTAAGGGCATCAC-3′). The amplifications were performed using EX Taq (TaKaRa Biotechnology, Tokyo, Japan) following cycles of 94°C for 30 s, 55°C for 30 s and 72°C for 30 s. Real-time RT PCR was performed with a SYBR Premix Ex TaqTM II Kit (TaKaRa Biotechnology, Tokyo, Japan) and the primer set of mDer-f2-FD/mDer-f2-RV according to the manufacturer’s instructions. The PCR program was as follows: initial polymerase activation for 10 s at 95°C; 40 cycles of 95°C for 5 s, followed by 60°C for 31 s. Analysis was by ABI PRISM 7000 Sequence Detection Systems (Applied Biosystems, CA, USA). Ubiquitin was selected as an internal control for normalization of real-time RT PCR data with the primers ubiquitin-FD (5′-AACCAGCTGAGGCCCAAGA-3′) and ubiquitin-RV (5′-ACGATTGATTTAACCAGTCCATGA-3′).
Analysis of mDer f 2 transcript 3′-ends
The mDer f 2 transcript 3′-ends were amplified using synthesized cDNAs as templates and a primer set of mDer-f2-FD/oligo-d(T)18. The PCR products were cloned into pT7/blue vector (TaKaRa Biotechnology, Tokyo, Japan), and positive clones were randomly selected and sequenced for confirmation of the mDer f 2 transcript 3′-ends.
Results
GluB-1 3′UTR increases accumulation of mDer f 2 in seed and leaf
The pUbiDerGluB and pUbiDerNos constructs were introduced into the rice genome by Agrobacterium-mediated transformation and 28 independent transgenic plants were regenerated with each construct. The presence of the mDer f 2 gene in each individual transgenic line was confirmed by PCR analysis: 27 lines with pUbiDerGluB and 26 with pUbiDerNos containing mDer f 2 were obtained (Table 1). Western blot analysis of transgenic seeds showed that mDer f 2 protein accumulated in 22 transgenic lines with pUbiDerGluB, but only in 6 with pUbiDerNos (Table 1). There were two bands with molecular masses of ca. 16–18 kDa that specifically immuno-reacted with anti-mDer f 2 antibody (Fig. 2a, lower panel). The upper band is likely to be a glycosylated form, since there is an N-linked glycosylation site in mDer f 2 at amino acid positions 73–75; the 16 kDa lower band is the unglycosylated form. To further compare levels of protein accumulation between pUbiDerGluB and pUbiDerNos lines, at least 4 positive seeds from each line were used to quantify mDer f 2 by immuno-dot-blot assay. Figure 2b shows that the pUbiDerGluB line accumulated approximately 4-fold more mDer f 2 than pUbiDerNos. The accumulation of mDer f 2 was also detected in leaf of pUbiDerGluB (Line 23), but not in roots of lines transformed with either construct (Fig. 2c).
Characterization of transgenic rice plants. a SDS-PAGE and Western blot analyses. Total proteins, extracted from non-transgenic wild-type (C), homozygous seeds of transgenic lines 23 and 27 (pUbiDerGluB) and lines 3 and 14 (pUbiDerNos), were separated by 12% SDS-PAGE (upper panel) and immuno-detected with anti-mDer f 2 antibody (lower panel). Molecular size markers are indicated on the right. b Histogram of the levels of accumulation of mDer f 2 in pUbiDerGluB and pUbiDerNos transgenic seeds. The mean for pUbiDerGluB was set as an arbitrary standard unit (1.0), and the horizontal bars indicate the average levels in each construct relative to this. One dot represents an average of four transgenic seeds. c Comparison of mDer f 2 accumulation in leaf (L), root (R) and seed (S) of pUbiDerGluB and pUbiDerNos transgenic plants
GluB-1 3′UTR regulates expression of mDer f 2 in a tissue-specific manner
To determine whether the greater accumulation of mDer f 2 in seed and leaf of pUbiDerGluB transgenic rice was due to the difference in concentration of mDer f 2 transcripts or translation efficiency, total RNAs were isolated from young leaf and developing seed of wt, pUbiDerGluB (line 23) and pUbiDerNos (line 14) plants, and subjected to RT-PCR and real-time RT-PCR analyses. Figure 3 shows that the level of expression of mDer f 2 transcripts in the leaf is essentially identical with either construct, while pUbiDerGluB has approximately 1.8-fold higher mDer f 2 transcript levels in seed than pUbiDerNos. These results indicate that the GluB-1 3′UTR may be involved in maintaining the stability and/or increasing the synthesis of mDer f 2 mRNA in transgenic seeds. By contrast, 3′UTRs of either gene had little effect on mDer f 2 transcript levels in leaves.
Expression of mDer f 2 in leaf and developing seed of transgenic rice. a RT-PCR analysis of mDer f 2 transcripts of non-transgenic wild-type (C), transgenic pUbiDerGluB line 23 and pUbiDerNos line 14. The level of 17S RNA expression was used as an internal control. Amplification cycles are indicated on the right. b Real-time RT-PCR analysis of mDer f 2 expression in non-transgenic wild-type (C), transgenic pUbiDerGluB line 23 and pUbiDerNos line 14. mDer f 2 expression levels are normalized to ubiquitin. n.d. not detected
GluB-1 3′UTR stabilizes mDer f 2 transcripts by limiting diversity of termination sites
To examine whether the GluB-1 and Nos 3′UTR affect stability of mDer f 2 mRNAs, polyadenylation [poly(A)] sites of these mRNAs isolated from leaf and seed of pUbiDerGluB (line 23) and pUbiDerNos (line 14) transgenic rice were analyzed (Fig. 4). Of 16 sequenced cDNA clones derived from mDer f 2 mRNAs containing GluB-1 3′UTR expressed in seed of the pUbiDerGluB transgenic line, 14 represented mRNAs terminated at 15 nt and the remaining two at 24 nt downstream from the 2nd poly(A) signal AATAAA (ie. 87.5% and 12.5% of total sequenced transcripts, respectively, Fig. 4). By contrast, among 15 sequenced mDer f 2 mRNAs expressed in leaf of the same transgenic line, poly(A) sites occurred in six different places. Of these, 7 were located at 15 nt, and 3 at 24 nt downstream from the 2nd poly(A) signal, the same sites as in seed (60.6% of total transcripts). The remaining four were found at different sites, with one at 35 nt downstream from the 1st poly(A) signal, and the others at 22, 29 and 127 nt downstream from the 2nd poly(A) signal. Taken together, these data indicate that mDer f 2 mRNA containing GluB-1 3′UTR was processed at two definitive sites in seed, while multiple sites were present in leaf (Fig. 4). It is notable that the poly(A) sites of mDer f 2 in transgenic seed are the same as in the native GluB-1 mRNAs (Accession Numbers: AK107343 and X15833). These results indicate that the GluB-1 3′UTR functions as an efficient transcriptional terminator in seed. In contrast, its role as a terminator may be dysregulated when expressed in other tissues such as leaves.
Identification of mDer f 2 transcript 3′-ends in seed and leaf of transgenic rice pUbiDerGluB and pUbiDerNos lines. a The partial cDNA sequences of 3′UTRs of GluB1 and Nos are shown and the consensus motifs of poly(A) signal AATAAA or AATAAT for mRNA 3′-end formation are in bold with a second underlined. The circled numbers above the cDNA sequence indicate different termination sites determined for mDer f 2 transcripts obtained from seed and leaf of pUbiDerGluB and pUbiDerNos. b The numbers of different 3′-ends of mDer f 2 transcripts are shown as percentages
Regarding the pUbiDerNos construct, the 3′-ends of the mDer f 2 transcript containing Nos 3′UTR are more divergent in both seed and leaf. Poly(A) sites of mDer f 2 occurred in 8 and 6 sites in seed and leaf, respectively (Fig. 4). About 60% of the 3′-ends of transcripts expressed in seed were located at 7–31 nt (7, 13, 19 and 31) downstream from the 1st poly(A) signal, the others at 8–45 nt (8, 27, 39 and 45) downstream from the 2nd poly(A) signal. In leaf, some cleaved at 14–19 nt downstream from the 1st poly(A) signal, the others at 24–45 nt (24, 27, 42 and 45) from the 2nd poly(A) signal. The polymorphic features of transcript 3′-ends in pUbiDerNos may affect the stability of the mDer f 2 mRNA in seed and leaf, which results in much lower accumulation of mDer f 2 in pUbiDerNos transgenic rice plants (Fig. 2).
Discussion
Gene expression is regulated both at the level of transcription and by posttranscriptional modification, the latter being a fundamental step in the processing of primary transcripts by capping of 5′-ends, splicing of introns and maturation of the 3′-end by endonucleolytic cleavage followed by addition of a poly(A) tail. The 3′UTRs and their poly(A) tails serve multiple facets of common cellular functions including transport of mRNA from the nucleus into cytoplasm, enhancement of mRNA stability, regulation of mRNA translation and controlling the development of the plant (Hunt 2008; Simpson et al. 2003).
When the GluB-1 terminator was used, mDer f 2 expressed under the control of the maize ubiquitin promoter accumulated in both seed and leaf of transgenic rice. Although mDer f 2 was expressed at similar levels in leaf of both pUbiDerGluB and pUbiDerNos transformants, higher levels of the mDer f 2 protein were observed when using pUbiDerGluB (Figs. 2c, 3). This suggests that GluB-1 3′UTR regulates mDer f 2 posttranscriptionally and/or translationally in the leaf. Interestingly, there was approximately 1.8-fold more mDer f 2 mRNA and on average 4-fold more mDer f 2 protein in transgenic seeds of pUbiDerGluB than pUbiDerNos lines (Figs. 2b, 3), indicating that the GluB-1 3′UTR regulates mDer f 2 accumulation at the transcriptional, posttranscriptional and/or translational level in seeds. At present, we still do not have an explanation for the differences between leaf and seed. One possibility is that higher expression of the pUbiDerGluB construct in seed is related to the origin of GluB-1 3′UTR itself in seed. It is reasonable to expect that the “native” cellular environment for GluB-1 3′UTR facilitates both greater expression of mRNA as well as greater translation of protein in seeds. The important influence of GluB-1 3′UTR on enhancement of accumulation of mDer f 2 protein in seed and leaf suggest that the production of recombinant proteins relying on high-level expression might benefit from using GluB-1 3′UTR, especially when seed is used for transgene product accumulation.
In plants, the poly(A) signals located in 3′UTRs seem to be more variable than in mammals and multiple poly(A) sites have been shown to occur in a tissue-specific manner (Hunt 2008; Shen et al. 2008). When polyadenylation generates the diversity of mature transcripts with different 3′-ends, it can also result in changes that may affect mRNA stability or translatability, because it is known that polyadenylation affects the stability of mRNA (Hunt 2008). The heterogeneity of mDer f 2 transcripts containing the GluB-1 or Nos 3′UTR in leaf and developing seed certainly reflect the origins of the genes as well as the characteristics of their 3′UTRs. The mDer f 2 transcript containing the discrete 103 and 112 nt GluB-1 3′UTR in seeds may stabilize the mRNA, resulting in much higher transcript concentration and consequently enhancement of translation (Figs. 2, 3). Poly(A) addition at these definitive sites might be responsible for stabilization of mRNA. By contrast, highly divergent 3′-ends of mDer f 2 containing the Nos 3′UTR in leaf and seed of the pUbiDerNos transgenic line may result in mRNA instability and low translatability. Alternatively, interactions of the 3′UTRs of the mRNA sequences with specific mRNA-binding proteins may have different major roles in posttranscriptional and translational regulation in leaf and seed. Higher levels of transcripts in pUbiDerGluB transgenic seeds suggest that seed-specific trans-factors binding to GluB-1 3′UTR may be involved in stabilizing the transcripts.
The yield of recombinant proteins in transgenic plants can be increased by optimizing parameters that determine transcription, posttranscription, translation and post-translational modification (Streatfield 2007; Takaiwa et al. 2008). Our data suggest that the choice of 3′UTR sequence is a significant factor for optimal accumulation of recombinant proteins. Although at present we do not know whether GluB-1 3′UTR is implicated in quantitative regulation of recombinant proteins in dicotyledonous plants, selection of an optimal 3′UTR will certainly improve accumulation of recombinant proteins in plant-based production systems.
References
Ali S, Taylor W (2001) The 3′ non-coding region of a C4 photosynthesis gene increases transgene expression when combined with heterologous promoters. Plant Mol Biol 46:325–333
Bevan M, Barnes WM, Chilton MD (1983) Structure and transcription of the nopaline synthase gene region of T-DNA. Nucleic Acids Res 11:369–385
Christensen AH, Quail PH (1996) Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res 5:213–218
De Jaeger G, Scheffer S, Jacobs A, Zambre M, Zobell O, Goossens A, Depicker A, Angenon G (2003) Boosting heterologous protein production in transgenic dicotyledonous seeds using Phaseolus vulgaris regulatory sequences. Nat Biotechnol 20:1265–1268
Depicker A, Stachel S, Dhaese P, Zambryski P, Goodman HM (1982) Nopaline synthase: transcript mapping and DNA sequence. J Mol Appl Genet 1:561–573
Goto F, Yoshihara T, Shigemoto N, Toki S, Takaiwa F (1999) Iron fortification of rice seed by the soybean ferritin gene. Nat Biotechnol 17:282–286
Hunt AG (2008) Messenger RNA 3′-end formation in plants. Curr Top Microbiol Immunol 326:151–177
Knirsch L, Clerch LB (2000) A region in the 3′ UTR of MnSOD RNA enhances translation of a heterologous RNA. Biochem Biophys Res Commun 272:164–168
Ortega JL, Moguel-Esponda S, Potenza C, Conklin CF, Quintana A, Sengupta-Gopalan C (2006) The 3′ untranslated region of a soybean cytosolic glutamine synthetase (GS1) affects transcript stability and protein accumulation in transgenic alfalfa. Plant J 45:832–846
Shen Y, Ji G, Haas BJ, Wu X, Zheng J, Reese GJ, Li QQ (2008) Genome level analysis of rice mRNA 3′-end processing signals and alternative polyadenylation. Nucleic Acids Res 36:3150–3161
Simpson GG, Dijkwel PP, Quesada V, Henderson I, Dean C (2003) FY is an RNA 3′ end-processing factor that interacts with FCA to control the Arabidopsis floral transition. Cell 113:777–787
Streatfield SJ (2007) Approaches to achieve high-level heterologous protein production in plants. Plant Biotechnol J 5:2–15
Takai T, Yokota T, Yasue M, Nishiyama C, Yuuki T, Mori A, Okudaira H, Okumura Y (1997) Engineering of the major house dust mite allergen Der f2 for allergen-specific immunotherapy. Nat Biotechnol 15:754–758
Takaiwa F, Kikuchi S, Oono K (1987) Rice glutelin gene family—a major type of glutelin mRNAs can be divided into two classes. Mol Gen Genet 208:15–22
Takaiwa F, Yang L, Yasuda H (2008) Health-promoting transgenic rice: application of rice seeds as a direct delivery system for bioactive peptides in human health. In: Hirano HY, Hirai A, Sano Y, Sasaki T (eds) Biotechnology in agriculture and forestry, vol 62. Springer-Verlag, Berlin, pp 357–373
Acknowledgments
This work was supported by research grant “Genomics and Agricultural Innovation, GMC0008” from the Ministry of Agriculture, Forestry and Fisheries of Japan to F. Takaiwa, a ‘Grant-in-Aid for JSPS fellows’ to Y. Wakasa and a ‘Grant-in-Aid for Young Scientists’ (19780011) to T. Kawakatsu.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, L., Wakasa, Y., Kawakatsu, T. et al. The 3′-untranslated region of rice glutelin GluB-1 affects accumulation of heterologous protein in transgenic rice. Biotechnol Lett 31, 1625–1631 (2009). https://doi.org/10.1007/s10529-009-0056-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-009-0056-8