Abstract
Biodiesel, a renewable alternative to fossil energy, has shown great prospects for global proliferation in the past decade. Lipase catalyzed transesterification for biodiesel production, as a biological process with many advantages has drawn increasing attention. As a by-product, glycerol accounts for about 10% w/w of biodiesel during the process of biodiesel production. As a result, the conversion of glycerol has become a common problem which has to be resolved if considering large amount of biodiesel production. Glycerol can be fermented into 1,3-propanediol, a high value added chemical with a promising future in the polymers, for example, polytrimethylene terephthalate, and also fermentation approaches for 1,3-propanediol production which have drawn more and more attention due to advantages such as relatively low investment, mild reaction conditions and using renewable sources as the starting materials. Based on the latest technology advancements in lipase-mediated transformation for biodiesel production, the aerobic fermentation technology and genetic engineering for 1,3-propanediol production, and the integrated production of 1,3-propanediol from crude glycerol could be a promising way to improve the profit of the whole process during biodiesel production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
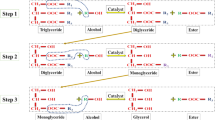
Biodiesel, defined as monoalkyl esters of long-chain fatty acid derived from triglycerides or renewable lipid resources (e.g. rapeseed oil, soybeen oil, palm oil, etc.) by transesterification or esterification with short-chain alcohols (Fig. 1), constitutes an environmentally friendly alternative to diesel. For it is able to be used directly in existing diesel engines without (or with little) modification, and is compatible with existing fuel distribution. As a result, the global biodiesel industry has grown significantly during the past decade.
Glycerol, as the principal by-product from biodiesel production units, is a low-cost renewable resource. With the rapid development of the biodiesel industry, an increasing surplus of glycerol is appearing in the world market. How to deal with the glycerol becomes a big problem. Currently, the glycerol is utilized as boiler fuel or the supplement for animal feed. When burned to produce thermal energy, the heating value of glycerol is slightly less than the current value of natural gas. However, the presence of ash generated by the salts and the water involved in the crude glycerol decreased the heating value significantly. On the other hand, crude glycerol is sold as a supplement for animal feed, but the market value of the latter is lower than the price of the former. With the development of biotechnology technology, 1,3-propanediol (1,3-PDO), a promising chemical as the monomer of many novel polymers such as polytrimethylene terephthalate (PTT), can be produced from crude glycerol by fermentation. PTT would need to exceed 11,250 million pounds to totally replace PET, so market demand for 1,3-PDO is expected to reach 500 million pounds, based solely on its use in PTT production (Johnson and Taconi 2007). For a long time, the production cost of 1,3-PDO depends much on the cost of substrate, which is a severe obstruction for the commercial production of 1,3-PDO by fermentation.
Since large quantities of low-cost crude glycerol is now available, conversion of glycerol into higher value-added products, especially 1,3-PDO, has become an important process for integration with biodiesel production.
Biodiesel production with enzymatic approaches
Applied prospect of biodiesel production
The first generation biodiesel markets in Europe and US have reached impressive biodiesel production capacity levels, but remain constrained by the availability of feedstock. In the BRIC nations of Brazil, Russia, India and China, key government initiatives are spawning hundreds of new opportunities for feedstock developments, biodiesel production and export. At present, the European Union (EU) accounts for 66% of the world’s total biodiesel production, followed by the United States at 1.5 billion liters (Agra Europe 2325, August 29, 2008). Total EU biodiesel production for 2007 was over 5.7 million metric tons, an increase of 16.8% from the 2006 figures, and production capacity for 2008 will reach 16 million metric tons (Calculation based on 330 working days per year, per plant.) (Fig. 2, European Biodiesel Board 2008). The US biodiesel production has increased from 946 million liters in 2006 to 1.70 billion liters in 2007, and has an estimate of 2.46 billion liters in 2008 (US National Biodiesel Board 2008). The world biodiesel production and capacity have increased from 7.1 million tons in 2006 to 9.0 million tons in 2007, and 12.2 million tons in 2006 to 23.1 million tons in 2007, respectively (Figs. 3, 4, Biodiesel 2020 2008). The world biodiesel market is estimated to reach 37 billion gal by 2016 growing at an average annual growth of 42% (Sims 2007). In the year 2007, there were only 20 oil producing nations supplying the needs of over 200 nations. By the year 2010, more than 200 nations will become biodiesel producing nations and suppliers. The world is entering a new era of participation by emerging market nations in global green energy production for transport fuels.
Lipase-mediated process for biodiesel production
Various routes have been proposed for both chemical and enzymatic processes of biodiesel production. Chemical processes, using alkali or acid as the catalysts, have been industrialized in biodiesel production in many countries. Recently, biodiesel production with enzymatic transesterification gained attention. Compared with chemical approaches, it conserves energy and easy to recover the byproduct glycerol, as no acidic or alkali catalysts have to be removed from the product, and free fatty acid (FFA) and water would not interfere with the reaction (Meher et al. 2006). Since feedstock with high FFA is feasible and the purification of fatty acid methyl esters is simple to accomplish, enzymatic processes have attracted more and more attention (Du et al. 2004).
Lipases from microorganisms like Mucor miehei, Rhizopus oryzae, Candida antarctica, and Pseudomonas cepacia are suitable for biodiesel formation (Du et al. 2007). In the case of lipase-mediated processes for biodiesel production, most efforts are made on immobilized extracellular lipases, for they are usually more stable than free lipases and can be used repeatedly without complex separation (Du et al. 2008). Various carriers, such as porous kaolinite, fiber cloth, hydrotalcite, macroporous resins, and silica gel, etc, have been applied to the lipases immobilization (Du et al. 2005; Yagiz et al. 2007). The Lipozyme TL IM immobilized on acyl migration in transesterification of soybean oil and hydrotalcite in transesterification of waste cooking oil have reached yields of 90% and 92.8%, respectively (Du et al. 2005; Yagiz et al. 2007).
Immobilized lipase-mediated transformation was firstly studied in a solvent-free system (Shimada et al. 1999). To solve the negative effects on the lipase activity caused by methanol and glycerol, Xu et al. (2004) studied stepwise methanol addition, such as three-step methanol addition, and found that the negative effect of methanol on lipase activity decreased. According to the non-aqueous enzymology principle, an enzyme could maintain high catalytic activity in hydrophobic organic solvents (usually LogP > 3, the higher the more hydrophobic), and in solvents such as n-hexane and petroleum ether, enzymes show relatively higher activity (Lara and Park 2004). So, organic solvent systems were introduced in immobilized lipase-mediated biodiesel production. However, though the enzymes are more active in the reaction, methanol and by-product glycerol are hardly soluble in the aforementioned hydrophobic organic solvents. Therefore, tert-butanol, a relatively hydrophilic organic solvent, in which both methanol and glycerol are soluble, has been developed as a novel reaction medium of lipase-mediated methanolysis for biodiesel production (Li et al. 2006). Though the LogP value of tert-butanol is only about 0.35, lipases still exhibit high activity and good stability in tert-butanol medium. The exploration on related mechanism has been carried out by Du et al. (2007), and LogP environment was proposed to consider the effect of whole environment on lipase activity instead of just considering the effect of organic solvent itself as traditionally calculated. The highest biodiesel yield of 95% could be obtained, and there was no obvious loss in lipase activity even after being re-used for 200 cycles with Lipozyme TL IM and Novozym 435 combined use in a tert-butanol system (Li et al. 2006). By using this novel process, the operational life of the immobilized lipase improved over 50-fold compared with traditional enzymatic approaches. After successful demonstration over 1 year in a pilot plant with a capacity of 200 kg/d biodiesel, the world’s first commercial facilities with a capacity of 20,000 t/year was constructed in Hunan, China and this was put into operation on December 8, 2006. The residual activity of the lipase is still more than 85% of its initial value after continuously running for more than 1 year. The second facility with a capacity of 200,000 t/year biodiesel will be operational by 2009 in Singapore. Royon et al. (2007) recommended lipase-mediated biodiesel production from cottonseed oil in tert-butanol medium. A methanolysis yield of 97% was observed, and no obvious decrease of ester yields was detected during continuous reactor operation over 500 h. The use of tert-butanol medium in biodiesel production system could solve the negative effects caused by methanol and glycerol existing in conventional reaction mediums, and shows significant prospect.
Using whole cells such as R. oryzae as the catalyst for biodiesel production is a potential way to reduce the cost of biocatalyst, which also can avoid the complex procedures of isolation, purification, and immobilization of conventional immobilized lipase preparation, though many matters such as scaling up, process optimization need to be investigated further (Zeng et al. 2006; Li et al. 2007, Fig. 5).
Integrated production of 1,3-PDO from by-product glycerol
As mentioned above, glycerol is a co-product at about 10% w/w of the esters during the biodiesel production. With the extensive application of biodiesel in a large commercial scale, plenty of the large supply of glycerol in the market needs to be effectively utilized in the near future. Integrated production of 1,3-PDO from glycerol could be a promising way to improve the profit of the whole process during biodiesel production (Fig. 6).
Over the past few decades, there has been growing interest in 1,3-PDO as an important organic chemical raw material. 1,3-PDO can be synthesized from petrochemicals by chemical approaches or from renewable substrates by microbial fermentation. However, it is well known that there are many disadvantages associated with chemical approaches for 1,3-PDO production, such as low selectivity, high temperature, high pressure needed, etc. Fermentation approaches for 1,3-PDO production have drawn increasing attention by considering its advantages over chemical method such as relatively low investment, mild reaction conditions and using renewable feedstock as the starting materials.
Microbial production of 1,3-propanediol
In the 1880s, a bacteria which can convert glycerol to 1,3-PDO was found, but only very recently, has its biotechnological significance been recognized, and more directed research initiated. Microbial fermentation for 1,3-PDO production from glycerol has already been extensively studied in species belonging to the family Klebsiella, Citrobacter, Clostridium, Enterobacter, and Lactobacillus (Zheng et al. 2008a). Klebsiella pneumoniae is the most wildly studied strain owing to its great performance as a 1,3-PDO producer. Crude glycerol, particularly form biodiesel plants, has been demonstrated to be an excellent fermentation feedstock for 1,3-PDO production. The main metabolic pathways of glycerol fermentation include oxidative and reductive pathways. In all bacteria able to ferment glycerol into 1,3-PDO, 1,3-PDO is produced by the reductive pathway in two successive enzymatic reactions. Glycerol dehydratase (GDHt), composed of three subunits dhaB-alpha, dhaB-beta and dhaB-gamma, catalyzes the dehydration reaction of glycerol into 3-hydroxypropionaldehyde (3-HPA), which is then reduced to 1,3-PDO by 1,3-propanediol oxidoreductase (PDOR) encoded by the gene dhaT with the consumption of reducing power NADH generated in the oxidation pathway (Fig. 7, Nakamura and Whited 2003).
The glycerol fed-batch fermentation of 1,3-PDO was firstly carried out under anaerobic conditions. As a more cost-effective process, micro-aerobic fermentation was then developed and extensively studied in the last decade. Zheng et al. (2008b) studied two-stage fed-batch fermentation of 1,3-PDO by k. pneumoniae under aerobic conditions, and 1,3-PDO concentration reached 74.07 g/l. The high 1,3-PDO yield and productivity of 0.62 mol/mol and 3.08 g/l/h was obtained respectively.
The application of metabolic engineering and recombinant DNA technology is expected to increase the yield and final concentration of 1,3-PDO. Much effort has been put to establish a profitable biotechnological production of 1,3-PDO based on glycerol. Kinetic and pathway analyses suggested that GDHt is a major rate-limiting enzyme for the production of 1,3-PDO in K. pneumoniae, especially at high glycerol concentrations (Ahrens et al. 1998). It has also been demonstrated that GDHt activity is a limiting step for 1,3-PDO production in C. butyricum (Abbad-Andaloussi et al. 1996). Therefore, increasing the dehydratase activity in the native producer K. pneumoniae was considered to be a likely effective way to increase the productivity of 1,3-PDO fermentation. However, the excessive expression of GDHt and/or PDOR was demonstrated to be not favorable for overproducing 1,3-PDO in the glycerol fermentation by K. pneumonia (Zheng et al. 2006), while over expression of PDOR in K. pneumoniae could reduce the accumulation of 3-HPA from 7.55 to 1.49 mmol/l (Hao et al. 2008). In the microbial fermentation of 1,3-PDO, several byproducts are formed, i.e., butanol by C. pasteurianum, butyric acid by C. butyricum, and 2,3-butanediol, succinic acid, lactate, ethanol, and acetate, by K. pneumoniae, K. oxytoca and C. freundii. In order to reduce the by-product synthesis, Yang et al. (2007) demonstrated fermentation of 1,3-propanediol by a lactate-deficient mutant of K. oxytoca under microaerobic conditions. The final concentration of 1,3-PDO reached 62.64 g/l using glycerol as single carbon resource, and the lactate could not be detected. In the batch-fed fermentation of a lactate-deficient k. pneumoniae under aerobic conditions, 1,3-PDO could reach 102 g/l, while lactate was less than 3.0 g/l, and similar results were obtained using crude glycerol as feedstock (Data not published). Though according to a patent of Dupont and Genencor (Nakamura and Whited 2003), a final concentration of 135 g/l 1,3-PDO was obtained with transformed E. coli using glucose as substrate, no more than 10 g/l of 1,3-PDO produced by the E. coli contained the enzymes GDHt and PDOR obtained from K. pneumoniae using glycerol as substrate (Wang et al. 2007). Up to now, thanks to metabolic engineering we have reached achievements to some extent, however, much effort is still needed to gain a more feasible and efficient microbial production of 1,3-PDO.
On the basis of existing knowledge and technology, a novel flexible process for 1,3-PDO production from crude glycerol or glucose by one or two steps was set up (Liu et al. 2005). The demonstration was achieved through a pilot plant and facility with a capacity of 4,000 t/year 1,3-PDO run by Hunan Rivers Bioengineering Company, China. The purity of final product 1,3-PDO is as good as 99.92%. Based on the above technology, it could be integrated with the production of biodiesel and 1,3-propanediol.
Conclusions
The global markets for biodiesel are entering a period of rapid, transitional growth, creating both uncertainty and opportunity. Integrated production of 1,3-PDO from by-product glycerol in biodiesel industry is a pathway to make the biodiesel industry more cost-effective and environmental-protective. Great achievements have enabled the commercialization of lipase-catalyzed transesterifiaction for biodiesel production and 1,3-PDO fermentation based on crude glycerol. However, many of these technologies still need additional research and development to make them more economically and operationally feasible for incorporation into existing biorefineries. For example, the operational life of the lipase could be prolonged greatly in some special reaction systems, and such achievements definitely will make lipase-mediated process more commercially attractive. In 1,3-PDO production, biological efforts including fermentation optimization of the natural glycerol-utilizing process and an ambitious metabolic engineering effort, directed towards more economical processes, will have impact upon reducing undesired by-products formation and achieving high product yield.
References
Abbad-Andaloussi S, Guedon E, Spiesser E, Petitdemange H (1996) Glycerol dehydratase activity: the limiting step for 1,3-propanediol production by Clostridium butyricum DSM 5431. Lett Appl Microbiol 22:311–314
Ahrens K, Menzel K, Zeng A, Deckwer W (1998) Kinetic, dynamic, and pathway studies of glycerol metabolism by Klebsiella pneumoniae in anaerobic continuous culture. III. Enzymes and fluxes of glycerol dissimilation and 1,3-propanediol formation. Biotechnol Bioeng 59:544–552
Du W, Xu YY, Liu DH, Zeng J (2004) Comparative study on lipasecatalyzed transformation of soybean oil for biodiesel production with different acyl acceptors. J Mol Catal B 30:125–129
Du W, Xu YY, Liu DH, Li ZB (2005) Study on acyl migration in immobilized lipozyme TL-catalyzed transesterification of soybean oil for biodiesel production. J Mol Catal B 37:68–71
Du W, Liu DH, Li LL, Dai LM et al (2007) Mechanism exploration during lipase-mediated methanolysis of renewable oils for biodiesel production in a tert-butanol system. Biotechnol Prog 23:1087–1090
Du W, Li W, Sun T, Chen X, Liu DH (2008) Perspectives for biotechnological production of biodiesel and impacts. Appl Microbiol Biotechnol 79:331–337
Hao J, Wang W, Tian JS, Li JL, Liu DH (2008) Decrease of 3-hydroxypropionaldehyde accumulation in 1,3-propanediol production by over-expressing dhaT gene in Klebsiella pneumoniae TUAC01. J Ind Microbiol Biotechnol 35:735–741
Johnson DT, Taconi KA (2007) The glycerin glut: options for the value-added conversion of crude glycerol resulting from biodiesel production. Environ Prog 26(4):338–348
Lara PV, Park EY (2004) Potential application of waste activated bleaching earth on the production of fatty acid alkyl esters using Candida cylindracea lipase in organic solvent system. Enzyme Microb Technol 34:270–277
Li LL, Du W, Liu DH, Wang L, Li ZB (2006) Lipase-catalyzed transesterification of rapeseed oils for biodiesel production with a novel organic solvent as the reaction medium. J Mol Catal B 43:58–62
Li W, Du W, Liu DH (2007) Rhizopus oryzae IFO 4697 whole cell catalyzed methanolysis of crude and acidified rapeseed oils for biodiesel production in tert-butanol system. Process Biochem 42:1481–1485
Liu DH, Liu HJ, Lin RH, Hao J (2005) A method for producing 1,3-propanediol by using the by-product glycerol of biodiesel production. Chinese patent ZL200510011867.8
Meher LC, Vidya Sagar D, Naik SN (2006) Technical aspects of biodiesel production by transesterification—a review. Renew Sustain Energy Rev 10:248–268
Nakamura CE, Whited GM (2003) Metabolic engineering for the microbial production of 1,3-propanediol. Curr Opin Biotech 14:454–459
Royon D, Daz M, Ellenrieder G, Locatelli S (2007) Enzymatic production of biodiesel from cotton seed oil using t-butanol as a solvent. Bioresour Technol 98:648–653
Shimada Y, Watanabe Y, Samukawa T, Sugihara A, Noda H, Fukuda H, Tominaga Y (1999) Conversion of vegetable oil to biodiesel using immobilized Candida antarctica lipase. JAOCS 76:789–793
Wang FH, Qu HJ, Zhang DW, Tian PF, Tan TW (2007) Production of 1,3-propanediol from glycerol by recombinant E-coli using incompatible plasmids system. Molecular Biotechnol 37(2):112–119
Xu YY, Du W, Zeng J, Liu DH (2004) Conversion of soybean oil to biodiesel fuel using lipozyme TL IM in a solvent-free medium. Biocatal Biotransform 22:45–48
Yagiz F, Kazan D, Akin AN (2007) Biodiesel production from waste oils by using lipase immobilized on hydrotalcite and zeolites. Chem Eng J 134:262–267
Yang G, Tian JS, Li JL (2007) Fermentation of 1,3-propanediol by a lactate deficient mutant of Klebiella oxytoca under microaerobic conditions. Appl Microbiol Biotechnol 73:1017–1024
Zeng J, Du W, Liu XY, Liu DH, Dai LM (2006) Study on the effect of cultivation parameters and pretreatment on Rhizopus oryzae cellcatalyzed transesterification of vegetable oils for biodiesel production. J Mol Catal B 43:15–18
Zheng P, Wereath K, Sun JB, van den Heuvel J, Zeng AP (2006) Overexpression of genes of the dha regulon and its effects on cell growth, glycerol fermentation to 1,3-propanediol and plasmid stability in Klebsiella pneumoniae. Process Biochem 41:2160–2169
Zheng ZM, Xu YZ, Liu HJ, Guo NN, Cai ZZ, Liu DH (2008a) Physiologic mechanisms of sequential products synthesis in 1,3-propanediol fed-batch fermentation by Klebsiella pneumoniae. Biotechnol Bioeng 100(5):923–932
Zheng ZM, Cheng KK, Hu QL, Liu HJ, Guo NN, Liu DH (2008b) Effect of culture conditions on 3-hydroxypropionaldehyde detoxification in 1,3-propanediol fermentation by Klebsiella pneumoniae. Biochem Eng J 39:305–310
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xu, Y., Liu, H., Du, W. et al. Integrated production for biodiesel and 1,3-propanediol with lipase-catalyzed transesterification and fermentation. Biotechnol Lett 31, 1335–1341 (2009). https://doi.org/10.1007/s10529-009-0025-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-009-0025-2