Abstract
To investigate the function of nuclear-localized plant HSP70, we used NtHSP70-1 isolated from Nicotiana tabacum. The subcellular localization of NtHSP70-1 was identified by fluorescence microscopy for NtHSP70-1/GFP or smGFP fusion proteins in onion epidermal cells, obtained using particle gun bombardment. To analyze the drought-stress tolerance and thermoprotective role of NtHSP70-1, we obtained transgenic tobacco plants that constitutively expressed elevated levels of NtHSP70-1 as well as transgenic plants containing either the vector alone or else having NtHSP70-1 in the antisense orientation. From analysis for genomic DNA in transgenic seedlings after heat stress, NtHSP70-1 helps to prevent the fragmentation and degradation of nuclear DNA during heat stress. In addition, seedlings constitutively overexpressing NtHSP70-1 grew to be healthy plants, whereas transgenic vector or antisense seedlings resulted in death after heat-/drought-stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because plants are constantly exposed to abiotic stresses, they must possess strong mechanisms adapting to various environmental stresses. One important mechanism is correlated with heat shock proteins (HSPs) that play a role as molecular chaperones (Seki et al. 2002; Hong et al. 2003; Sung and Guy 2003; Takahashi et al. 2004; Cho and Hong 2006). HSPs as stress-inducible genes respond to environmental abiotic stresses such as high-/low-temperature, drought and high salinity (Sung et al. 2001; Sung and Guy 2003; Cho and Hong 2004). Stress-inducible genes are related to two pathways: one directly protecting against environmental stresses; and the other regulating gene expression and signal transduction in the stress response (Hasegawa et al. 2000; Seki et al. 2002). In addition, stress-inducible genes have been used to improve the stress tolerance by gene transfer. Therefore, it is important to analyze the functions of HSPs to understand the molecular mechanisms of stress response and to improve the stress tolerance of organisms by gene manipulation. Among the HSPs, HSP70 is well known as a logical model for studying the adaptation mechanism (Mayer et al. 2001; Hartl and Hayer-Hartl 2002). HSP70 together with co-chaperones and co-factor contributed to the protection of protein substrates from high temperature. However, the mode of HSP70 action correlating to molecular chaperones has been elucidated mainly in Escherichia coli and mammalian cells. Recently, HSP70 was reported as a molecular chaperone responding in plants during abiotic stress exposure (Sung et al. 2001; Sung and Guy 2003; Cho and Hong 2004) and was associated with diverse environmental stresses. This means that HSP70 may function in similar stress-response pathways under different stress conditions. This combined effect may indicate a protective role for HSP70 against simultaneous stresses and may explain the documented phenomenon of “cross tolerance” (Bowler and Fluhr 2000; Rizhsky et al. 2002). However, it has not been investigated that a combination of stresses was applied simultaneously to plants.
In most organisms, HSP70s are encoded by gene families and particularly many gene members for HSP70 have been identified in plants (Sung et al. 2001; Cho and Hong 2004). The diversity of those HSP70 members may be a significant basis for the multifunctional HSP70 family. Since HSP70s were first characterized in plants, they have been discovered in diverse subcellular compartments, such as the cytoplasm, chloroplast, mitochondrion and endoplasmic reticulum (ER) (Lee and Schöffl 1996; Schroda et al. 1999; Mackenzie and Mclntosh 1999; Alvim et al. 2001; Sung et al. 2001). According to these reports, each HSP70 functioned differently in each subcellular organelle. However, a nucleus-localized HSP70 has not yet been characterized in plants. In the present study, we have demonstrated that NtHSP70-1 is putatively localized in the nucleus. In addition, NtHSP70-1 may induce thermotolerance and drought-stress resistance to plants by relationship with pathways protecting nucleus under stress.
Material and methods
Plasmid constructs for subcellular localization of NtHSP70-1
The ORF of NtHSP70-1 was PCR-amplified by denaturation at 94°C for 40 s, annealing at 60°C for 40 s, and extension at 72°C for 2 min 30 s (30 cycles). The forward primer, 5′-AAAGGATCC ATGGCTCCCGCCGTCGG-3′ (BamHI site underlined and NtHSP70-1 start codon in bold), and the reverse primer, 5′-AAAGGATCCTGTCGACCTCCTCGACGG-3′, were used in the PCR. The amplified PCR product was then digested with BamHI and ligated into the smGFP vector (Hong and Hwang 2005; Lee et al. 2006) at the BamHI site to locate NtHSP70-1 under the control of the cauliflower mosaic virus (CaMV) 35S promoter in the sense orientations. The binary vector, plasmid smGFP, in which the cauliflower mosaic virus (CaMV) 35S promoter drives expression of GFP, contains a gene-soluble modified green fluorescent protein (smGFP). A BamHI fragment encoding NtHSP70-1 was cloned into the corresponding site on smGFP to create plasmid NtHSP70-1/smGFP in which NtHSP70-1 was fused to the 5′-end of GFP; the NtHSP70-1/smGFP in-frame fusion was confirmed by nucleotide sequence analysis. This recombinant DNA was transformed into E. coli strain DH5α.
To produce an NtHSP70-1/smGFP expression construct without introns included in NtHSP70-1, an inverse polymerase chain reaction was performed. The forward primer, 5′-ATGTGATATCATCGCCAACG-3′, and the reverse primer, 5′-TTACCGATCTCACGGAAGAT-3′, in the first intron, and the forward primer, 5′-AAAGATCTTAGCACCAACGT-3′, and reverse primer, 5′-CTTATGTTTGCGCTTGAACT-3′, in the second intron, were used for inverse PCR. PCR products were self-assembled with T4 DNA ligase (Invitrogen, Carlsbad, CA, USA).
Transient expression in onion epidermal cells
Particle bombardment with a Biolistic PDS-1000/He system (Bio-Rad, Hercules, CA, USA) was used to introduce GFP fusion plasmids into onion epidermal cells. Tungsten particles were coated with the plasmid NtHSP70-1/GFP or smGFP (the vector), and a helium pressure of 1100 psi was employed. Tungsten particles, 500 μg, coated with 0.8 μg DNA was used in each shot. The target distance between the stop screen and onion piece was set at 9 cm. Onions (Allium cepa L.) used for particle bombardment were obtained from a local supermarket. After bombardment, onion pieces were placed on MS medium and kept in darkness at 22°C for 15 h. The onion epidermal cells were then examined under a fluorescence microscope (Nikon, Tokyo, Japan) or a Radiance 2100 Confocal Laser Scanning Microscope System (Bio-Rad), and single optical sections were scanned to visualize the resulting images for each transient expression.
Construction of a plant expression vector with NtHSP70-1 and transformation of tobacco
Construction of a plant expression vector with NtHSP70-1 and transformation of tobacco were performed as previously described by Cho and Hong (2006). Putative transgenic plants were then transplanted into soil and grown in an insulated greenhouse, with a double door, at 24–25°C under natural light, with supplementary fluorescent lamps to maintain a 16 h photoperiod. Those transgenic plants (T0 plants) carrying the recombinant construct of NtHSP70-1 in the sense or antisense orientation, as well as those containing only the vector, were self-fertilized (T1 seeds).
Extraction of RNA and protein and analysis of Northern and Western blot
Total RNA and protein were extracted from transgenic plants and Northern and Western blotting were performed by the method described by Cho and Hong (2006). RNA blot analysis for putative transgenic plants carrying ORF of NtHSP70-1 in sense or antisense orientation was carried out with α32P-dUTP labelled antisense or sense NtHSP70-1 riboprobe. Hybridization was performed in a solution containing 0.5 M sodium phosphate (pH 7.2), 7% (w/v) SDS and 1 mM EDTA (pH 8.0) at 68°C. Immunoblot analysis was performed with a commercial anti-human HSP70 (HSC70) diluted 1:3000 in 5% (w/v) nonfat milk in TBS.
Genomic DNA extraction and analysis of oligonucleosomal fragmentation
Three-week-old T1 transgenic seedlings constitutively expressing NtHSP70-1, transgenic seedlings with NtHSP70-1 in antisense orientation, or transgenic seedlings with the vector only resistant to kanamycin were transplanted to Petri dishes containing the same amount of water. Selected three kinds of transgenic seedlings were treated at 45°C for 2 h in the light and were placed under continuous fluorescent lighting of 150 μmol m−2 s−1 at 24–25°C. After 0, 6 or 12 h, genomic DNA was extracted from the seedlings. One μg extracted genomic DNA subjected to electrophoresis on a 2% agarose gel and blotted onto a nylon membrane. The membrane was prehybridized for 1–2 h and hybridized for 16–22 h in a solution containing 0.5 M sodium phosphate (pH 7.2), 7% (w/v) SDS and 1 mM EDTA (pH 7.0) at 65°C. Tobacco genomic DNA labeled with 32P-dCTP using Prime-a-Gene system (Promega, USA) was used for oligonucleosomal fragmentation analysis. After washing in 2× SSPE and 0.1% SDS at 65°C for 30 min followed by 1× SSPE and 0.1% SDS at 65°C for 30 min, the membrane was exposed to an X-ray film (Kodak, USA) with two intensifying screens (Dupont, USA) at −75°C.
Measurement of chlorophyll accumulation
Seedlings treated with high temperature were placed under continuous fluorescent lighting of 150 μmol m−2 s−1 at 24–25°C. And then, the content of chlorophyll was measured after 10 days. In brief, 5 mg of seedlings were soaked in 1 ml ethanol and kept in the dark for 24 h. Chlorophyll leaked out from the seedlings into ethanol and 500 μl ethanol extracts were taken from each sample for the measurement of absorbance. Absorbance of the extract was scanned from 400 nm to 750 nm. Chlorophyll content was calculated using a set of equations: \( {\text{Chl }}a\,\left( {{\text{mg}}/{\text{l}}} \right) = 13.7{\text{ A}}665 - 5.76{\text{A}}649; \) \( {\text{Chl }}b\,\left( {{\text{mg}}/{\text{l}}} \right) = 25.8{\text{ A}}649\,-\,7.6{\text{ A}}665; \) \( {\text{Chl }}a + b\,\left( {{\text{mg}}/{\text{l}}} \right) = 6.1{\text{ A}}665 + 20.04{\text{ A}}649. \)
Thermotolerance and drought-stress resistance of transgenic seedlings
Experiments to analyze thermotolerance were conducted in controlled environmental cabinets and using seedlings for three distinct transgenic tobacco genotypes: transformed control (pBKS1-1 vector alone), transformed sense (35S-NtHSP70-1 S lines), and antisense (35S-NtHSP70-1 AS lines). Three-week-old tobacco seedlings resistant to kanamycin were transplanted to Petri dishes and moistened with equal amounts of water. Fifty seedlings that were resistant to kanamycin for control, sense, or antisense were exposed to light at 45°C for 2 h. After heat treatments, the seedlings were maintained at 24–25°C, and viability was assessed on a daily basis for as long as 10 days. The results were documented photographically and statistically. Experiments to analyze water-stress tolerance were performed as previously described by Cho and Hong (2006).
Results
Putative localization of NtHSP70-1/GFP in the nucleus
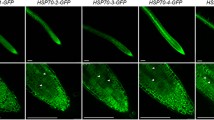
To demonstrate the subcellular localization of NtHSP70-1 in vivo, the localization of NtHSP70-1-derived GFP fusions was investigated (Fig. 1). GFP was expressed under the CaMV 35S promoter (Fig. 1a). The construct NtHSP70-1/GFP or the vector smGFP was introduced in onion epidermal cells by particle bombardment. After 15 h in darkness at 22°C, onion pieces placed on MS medium were analyzed under a fluorescence microscope (Fig. 1b). In onion epidermal cells expressing NtHSP70-1/GFP, fluorescence was observed only in the nucleus (Fig. 1b right). In contrast, onion cells expressing only the smGFP showed fluorescence throughout the cell (Fig. 1b left). The nuclear fluorescence of smGFP is due to the small size (28 kDa) of the GFP; molecules of less than 40–60 kDa are required for possible passive diffusion through nuclear pore complexes (Nagegowda et al. 2005). By these results, we demonstrated that NtHSP70-1 accumulates in the nucleus.
Construction of NtHSP70-1::GFP fusion (a) and fluorescent images of smGFP and NtHSP70-1::GFP fusion proteins transiently expressed in onion epidermal cells (b). Particle bombardment was performed to introduce plasmids into onion epidermal cells with a Biolistic PDS-1000/He particle delivery system. Tugsten particles (1.1 μm) and 1100 psi helium pressure were used. After bombardment, onion pieces were kept in darkness at 24°C for 18 h. In the control (left in b), onion cells were bombarded with tungsten particles coated with vector smGFP cDNA. Subsequently, bombarded tissues were examined under a fluorescence microscope. CaMV35S, cauliflower mosaic virus (CaMV) 35S promoter; GFP, green fluorescent protein; NOS, nopaline synthase terminator
Generation of plants with altered HSP70 levels and quantification of HSP70 expression
To identify the function of nuclear-localized NtHSP70-1, we generated and selected transgenic tobacco plants as previously described by Cho and Hong (2006). Selected independent transgenic lines were screened to evaluate HSP70 levels by immunoblotting and RNA blotting (Fig. 2b, c). RNA blot analyses for the transgenic tobacco plants confirmed constitutive expression of NtHSP70-1 transcript (S4, 11, 12, and 22) and antisense NtHSP70-1 transcript (AS14, 21, 35, and 43) when tissues were exposed to a standard temperature of 24–25°C (Fig. 2b). Under normal, non-stressed conditions, leaf levels of the tobacco HSP70 protein, NtHSP70-1, were significantly higher than from those of wild-type control and the pBKS1-1-transformed control and antisense transgenic leaves (Fig. 2c). For comparison with our nontransgenics, we selected two transgenic lines for three different transgene constructs (S12, S22, V4, V5, AS21, AS35), using plants with no noticeable differences in their growth rates.
Confirmation of NtHSP70-1 expression in transgenic tobacco plants. Total RNAs from putative transgenic plants were extracted and separated in a formaldehyde-agarose gel and then was stained with Ethidium bromide. Blots were hybridized to 32P-dUTP labelled antisense or sense NtHSP70-1 riboprobe. c Proteins were analyzed on a 12% SDS-PAGE gel and stained with Coomassie Brilliant Blue. Protein blot analysis for NtHSP70-1 produced in transgenic plants under non-heat-stressed conditions with a commercial anti-human HSP70 (HSC70). The transcript size for NtHSP70-1 was 3 kb and the molecular mass was estimated at 71.01 kDa. Vector, transgenic plants carrying only the vector; Sense, transgenic plants with NtHSP70-1 in sense orientation; Antisense, transgenic plants with NtHSP70-1 in antisense orientation
NtHSP70-1 is related to protect the degradation and cleavage of nuclear DNA into oligonucleosomal fragments induced by heat stress
To investigate the functions of nuclear-localized NtHSP70-1, 3-week-old kn-resistant T1 seedlings were treated at 45°C for 2 h. After heat treatment, seedlings kept for 0, 6 and 12 h in non-stressed condition were used for extraction of genomic DNA. For comparing three kinds of transgenic seedlings (sense, antisense, or vector transgenic tobacco seedling) of DNA fragmentation and degradation, Southern blotting for genomic DNA was performed. Genomic DNA extracted from transgenic tobacco seedlings carrying only the vector (V) and 35S-NtHSP70-1 antisense transgenics (AS) showed the severe degradation and a ladder of DNA fragments that differed by just under 200 bp when run on an agarose gel. While, genomic DNA extracted from transgenic tobacco seedlings over-expressing NtHSP70-1 (sense, S) showed no ladder or weaker fragmentation and degradation than vector and antisense transgenic seedlings after heat stress. An addition, the degradation and cleavage of nuclear DNA into oligonucleosomal fragments were severed followed by 6 and 12 h in the antisense or vector transgenic tobacco seedlings but, the patterns of nuclear DNA from sense transgenic tobacco seedlings were slowly progressed after heat stress (Fig. 3).
Southern blot analysis of genomic DNA showing internucleosomal cleavage. DNA cleavage into oligomer-size fragments, a characteristic of some apoptotic cell death events in animal cells, was detected in tobacco plant under heat stress. a One microgram of extracted genomic DNA subjected to electrophoresis on a 2% agarose gel and was stained with ethidium bromide. Lanes 1–3 represent 0, 6 and 12 h. a Period for incubation at nonstress condition. b, c Southern blots were hybridized to tobacco genomic DNA labeled with 32P-dCTP. Lanes 1–3 represent 0, 6 and 12 h. b Period for incubation at nonstress condition. c Period for incubation at nonstress condition after heat stress for 2 h at 45°C. V, transgenic plants containing pBKS1-1 vector only; S, transgenic plants containing NtHSP70-1 in sense orientation in the vector; AS, transgenic plants containing NtHSP70-1 in antisense orientation in the vector
NtHSP70-1 is associated with thermotolerance
Transgenic tobacco plants that over-expressed NtHSP70-1 maintained their stress-tolerant phenotype under conditions of high temperature. To demonstrate this response, we transplanted 3-week-old kn-resistant T1 seedlings to Petri dishes containing the same amount of water, and then subjected to a 45°C heat shock for 2 h. The plants were then maintained at 24–25°C and their viability was assessed on a daily basis and was statistically and photographically recorded (Table 1, Fig. 4).
Thermotolerance of NtHSP70-1 over-expressing transgenic tobacco seedlings. Heat stress (at 45°C for 2 h) was induced in kn-resistant 3-week-old seedlings (all at same developmental stage) of pBKS1-1-transformed controls and 35S-NtHSP70-1-transformed tobacco plants in sense or antisense orientation. a Non-heat stressed 3-week-old seedlings. b Non-heat stressed 3-week-old seedlings were kept at 24–25°C for 2 weeks. c, d Heat stressed 3-week-old seedlings were kept at 24–25°C for 2 weeks. V, transgenic seedlings carrying only the vector; S, transgenic seedlings with NtHSP70-1 in sense orientation; AS, transgenic seedlings with NtHSP70-1 in antisense orientation
Effect of NtHSP70-1 in preventing chlorophyll breakdown under heat stress was determined in transgenic tobacco seedlings. Transgenic tobacco seedlings either carrying NtHS70-1 in sense or antisense orientation and the vector only were exposed to 45°C for 2 h and were maintained at 24–25°C in light. The chlorophyll content was then measured after 10 days. Chlorophyll content in the sense transgenic seedlings was approximately 3.5 times and 3 times higher than the chlorophyll content in the antisense and the vector transgenic seedlings (Table 1). Measurement of the content of chlorophyll indicated that transgenic tobacco seedlings with the NtHSP70-1 in sense orientation apparently showed significant effect in preventing chlorophyll breakdown compared with the antisense or the vector transgenic seedlings.
Constitutive NtHSP70-1 expression also provides a growth advantage to heat-stressed plants (Fig. 4). After 2 weeks, there was no meaningful difference in the phenotypes of plant under non-heat stressed condition (Fig. 4a, b). But, most of the heat-stressed seedlings with the antisense gene construct and the vector only exhibited leaf yellowing or some delay in growth and led to death. Whereas seedlings with the constitutively expressed NtHSP70-1 grew as green or healthy plants (Fig. 4c, d). On the basis of the level of chlorophyll accumulation, transgenic tobacco seedlings with the NtHSP70-1 in-sense orientation showed a significant level of thermotolerance compared with the antisense transgenic seedlings and transgenic seedlings carrying only the vector.
NtHSP70-1 is indirectly associated with water-stress tolerance
Transgenic tobacco plants that over-expressed NtHSP70-1 maintained their stress-tolerant phenotype under conditions of water deficit. To demonstrate this response, we transplanted 3-week-old kn-resistant T1 seedlings to Petri dishes containing the same amount of water, and then grew them with a normal water supply. The overexpression seedlings had smaller stature compared with empty vector-transformed or antisense transgenic seedlings. However, this difference became inapparent at 3 weeks after imbibition (Fig. 5). Seedlings were kept dry for 2 weeks. An increased correlation for the effects of NtHSP70-1 was evident in the stressed sense plants (S12, S22), whereas the pBKS1-1 transformed control plants (V4, V5) and the antisense plants of NtHSP70-1 (AS35, AS43) showed a wilted phenotype under water stress (Fig. 5).
Drought tolerance of NtHSP70-1 overexpressing transgenic tobacco plants. Water stress was induced in 3-week-old seedlings (all at same developmental stage) of pBKS1-1-transformed controls and 35S-NtHSP70-1-transformed tobacco plants in sense or antisense orientation by withholding irrigation for 2 weeks. a We transplanted 3-week-old kn-resistant T1 seedlings to Petri dishes containing the same amount of water, and then grew them with a normal water supply. b, c Elevated levels of NtHSP70-1 conferred drought tolerance to plants
When water was withheld from 3-week-old seedlings in Petri dishes for 2 weeks, this progressive drought resulted in a water stress-tolerant phenotype of sustained leaf turgidity that was manifested only by the transgenic plants over-expressing the NtHSP70-1 gene. In contrast, the leaves of pBKS1-1-transformed controls and 35S-NtHSP70-1 AS antisense transgenics were wilted. In addition, the leaves of the severely drought-stressed transgenic antisense seedlings and those that contained only the vector were dark brown compared with the normal green coloring of the stressed transgenic sense seedlings. Prolonged drought inhibited growth completely, such that the 35S-NtHSP70-1 AS antisense transgenic plants and pBKS1-1 transformed control plants eventually died.
Discussion
Nuclear HSP70 was first reported in yeast, mouse and human cells (Nollen et al. 2001; Tsukahara and Maru 2004) but not plants. Here, we selected NtHSP70-1 to investigate the functions of nuclear HSP70 in plant. For the analysis of the subcellular localization of NtHSP70-1 in plant cells, several constructs transiently or permanently overexpressing NtHSP70-1 were created. In onion epidermal cells, NtHSP70-1/GFP-fused green fluorescent protein (GFP) was detected in the nucleus (Fig. 1). According to study for the reported nuclear HSP70s, they mediate diverse nuclear processes, including protein synthesis, ribosomal RNA synthesis, assembly of ribosomal precursor particles and protein folding disrupted by heat stress, and the translocation of substrates across nuclear membranes (Nollen et al. 2001). These results mean that nuclear HSP70s are associated with repair of heat-induced nuclear damage and protection nuclear substrates and structure against heat stress. However, the functional role of plant nuclear HSP70 in nucleus under heat shock remains unknown.
In the present study, the analysis for nuclear DNA fragmentation in plant under heat stress is molecular level experiment demonstrating the function of NtHSP70-1 as nuclear HSP70 protecting nucleus. The protection of nuclear DNA fragmentation in plant over-expressing NtHSP70-1 after heat stress was prominent in comparison of nuclear DNA fragmentation from vector control and antisense plants in NtHSP70-1 expression (Fig. 3). In addition, transgenic seedlings overexpressing NtHSP70-1 had thermoprotective activity (Table 1, Fig. 4). A large amount of chlorophyll content remained in transgenic sense lines after heat stress means thermotolerance in plant, whereas chlorophyll loss is one of the major symptoms of leaf senescence. Nuclear DNA fragmentation was, though, detectable during subsequent senescent stages and leaf senescence leading to cell death (Ito and Fukuda 2002; Lee and Chen 2002; Serafini-Fracassini et al. 2002; Langston et al. 2005). Therefore, NtHSP70-1 may contribute to defense mechanism related to cell death because of protecting nucleus during heat stress. However, NtHSP70-1 seems not to act directly in protecting nuclear DNA fragmentation but likely acts upstream in the stress damage pathway by protecting heat induced protein damage in the nucleus (Nollen et al. 2001). Although this hypothesis must be confirmed through more experiments, this study has produced the important finding of plant nuclear HSP70 related with protecting nuclear DNA during heat stress.
Under drought, expression of HSPs have been detected through cDNA microarrays (Seki et al. 2002; Oono et al. 2003; Takahashi et al. 2004). These reports suggest that HSPs confer drought tolerance on plants. In our laboratory, research on over-expression of NtHSP70-1 in drought-stressed tobacco seedlings indicated that HSP70 was associated with drought tolerance in vivo (Cho and Hong 2006). However, whether HSP70 can induce both thermotolerance and drought-resistance to plants is unknown. In this study, we practiced drought tolerance with transgenic sense lines having thermotolerance activity. Under severe water-deficit conditions, their transgenic sense seedlings showed better growth than did either the transgenic antisense seedlings or those containing only the vector (Fig. 5). Expression of heat stress-/drought-induced genes are required under heat-/drought-stress conditions (Richard et al. 2000; Xiong and Zhu 2002; Oono et al. 2003). A combination of drought and heat shock can represent the conditions encountered by many plants and crops growing within arid and semiarid environments (Mittler et al. 2001). Therefore, this study may be applied for the development of new strategies and tools to enhance stress tolerance via genetic manipulations. In antisense transgenic plants treated heat stress and drought, the results similar to control suggested the indirect functions of NtHSP70-1 in defense mechanisms. Although NtHSP70-1 did not play a central role on thermoprotection and drought-stress tolerance, the gain of function for nuclear-localized NtHSP70-1 could be exactly observed in this study.
References
Alvim FC, Carolino SM, Cascardo JC, Nunes CC, Martinez CA, Otoni WC, Fontes EP (2001) Enhanced accumulation of BiP in transgenic plants confers tolerance to water stress. Plant Physiol 126:1042–1054
Bowler C, Fluhr R (2000) The molecular basis of cross tolerance. Trends Plant Sci 5:241–246
Cho EK, Hong CB (2004) Molecular cloning and expression pattern analyses of heat shock protein 70 genes from Nicotiana tabacum. J Plant Biol 47:149–159
Cho EK, Hong CB (2006) Overexpression of tobacco NtHSP70-1 contributes to drought-stress tolerance in plants. Plant Cell Rep 25:349–358
Hartl FU, Hayer-Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852–1858
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Hong JK, Hwang BK (2005) Functional characterization of PR-1 protein, β-1, 3-glucanase and chitinase genes during defense response to biotic and abiotic stresses in Capsicum annuum. Plant Pathol J 21:195–206
Hong SW, Lee U, Vierling E (2003) Arabidopsis hot mutants define multiple functions required for acclimation to high temperatures. Plant Physiol 132:757–767
Ito J, Fukuda H (2002) ZEN1 is a key enzyme in the degradation of nuclear DNA during programmed cell death of Tracheary elements. Plant Cell 14:3201–3211
Langston BJ, Bai S, Jones ML (2005) Increases in DNA fragmentation and induction of a senescence-specific nuclease are delayed during corolla senescence in ethylene-insensitive (etr1–1) transgenic petunias. J Exp Bot 56:15–23
Lee RH, Chen SCG (2002) Programmed cell death during rice leaf senescence is nonapoptotic. New Phytol 155:25–32
Lee JH, Schöffl F (1996) An Hsp70 antisense gene affects the expression of HSP70/HSC70, the regulation of HSF, and the acquisition of thermotolerance in transgenic Arabidopsis thaliana. Mol Gen Genet 252:11–19
Lee BJ, Park CJ, Kim SK, Kim KJ, Paek KH (2006) In vivo binding of hot pepper bZIP transcription factor CabZIP1 to the G-box region of pathogenesis-related protein 1 promoter. Biochem Biophys Res Commun 344:55–62
Mackenzie S, Mclntosh L (1999) Higher plant mitochondria. Plant Cell 11:571–585
Mayer MP, Brehmer D, Gassler CS, Bukau B (2001) Advances in protein chemistry: protein folding in the cell, vol 59. Academic Press, San Diego, pp 1–44
Mittler R, Merquiol E, Hallak-Herr E, Rachmilevitch S, Kaplan A, Cohen M (2001) Living under a “dormant” canopy: a molecular acclimation mechanism of the desert plant Retama raetam. Plant J 25:407–416
Nagegowda DA, Ramalingam S, Hemmerlin A, Bach TJ, Chye ML (2005) Brassica juncea HMG-CoA synthase: localization of mRNA and protein. Planta 221:844–856
Nollen EAA, Salomons FA, Brunsting JF, Van der Want JJL, Sibon OCM, Kampinga HH (2001) Dynamic changes in the localization of thermally unfolded nuclear proteins associated with chaperone-dependent protection. Proc Natl Acad Sci USA 98:12038–12043
Oono Y, Seki M, Nanjo T, Narusaka M, Fujita M, Satoh R, Satou M, Sakurai T, Ishida J, Akiyama K, Iida K, Maruyama K, Satoh S, Yamaguchi-Shinozaki K, Shinozaki K (2003) Monitoring expression profiles of Arabidopsis gene expression during rehydration process after dehydration using ca. 7000 full-length cDNA microarray. Plant J 34:868–887
Richard S, Morency MJ, Drevet C, Jouanin L, Séguin A (2000) Isolation and characterization of a dehydrin gene from white spruce induced upon wounding, drought and cold stresses. Plant Mol Biol 43:1–10
Rizhsky L, Liang H, Mittler R (2002) The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol 130:1143–1151
Seki M, Narusaka M, Ishida J, Nanjo T, Fujita M, Oono Y, Kamiya A, Nakajima M, Enju A, Sakurai T, Satou M, Akiyama K, Taji T, Yamaguchi-Shinozaki K, Carninci P, Kawai J, Hayashizaki Y, Shinozaki K (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J 31:279–292
Serafini-Fracassini D, Duca SD, Monti F, Poli F, Sacchetti G, Bregoli AM, Biondi S, Mea MD (2002) Transglutaminase activity during senescence and programmed cell death in the corolla of tobacco (Nicotiana tabacum) flowers. Cell Death Differ 9:309–321
Sung DY, Guy CL (2003) Physiological and molecular assessment of altered expression of Hsc70-1 in Arabidopsis: evidence for pleiotropic consequences. Plant Physiol 132:979–987
Sung DY, Vierling E, Guy CL (2001) Comprehensive expression profile analysis of the Arabidopsis HSP70 gene family. Plant Physiol 126:789–800
Takahashi S, Seki M, Ishida J, Satou M, Sakurai T, Narusaka M, Kamiya A, Nakajima M, Enju A, Akiyama K, Yamaguchi-Shinozaki K, Shinozaki K (2004) Monitoring the expression profiles of genes induced by hyperosmotic, high salinity, and oxidative stress and abscisic acid treatment in Arabidopsis cell culture using a full-length cDNA microarray. Plant Mol Biol 56:29–55
Tsukahara F, Maru Y (2004) Identification of novel nuclear export and nuclear localization related signals in human heat shock cognate protein 70. J Biol Chem 279:8867–8872
Xiong L, Zhu JK (2002) Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ 25:131–139
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, E.K., Choi, Y.J. A nuclear-localized HSP70 confers thermoprotective activity and drought-stress tolerance on plants. Biotechnol Lett 31, 597–606 (2009). https://doi.org/10.1007/s10529-008-9880-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-008-9880-5