Abstract
The gene dexYG encoding the dextransucrase from an industrial strain of Leuconostoc mesenteroides 0326 was isolated by PCR. The nucleotide sequence of the dexYG gene consists of an open reading frame (ORF) of 4,584 bp, coding for a 1,527 aa protein with a Mr of 170 kDa. The results were analysed by a BLAST similarity search of the GenBank database, which revealed the amino acid sequence was similiar to dsrD derived from L. mesenteroides Lcc4. The dexYG gene was subcloned into the plasmid pET28a(+) and was expressed in E. coli BL21 (DE3) by IPTG induction. The pH value was one of the main reasons which caused the degradation of enzyme activity in the later stage of induction. The highest activity was reached 36 U/ml after 5 h induction in medium at pH 6.0. Biotransformation yield of the enzyme reached 65% and the molecular weight of transformed dextran was more than 68 kDa in 2 h.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dextransucrase (EC 2.4.1.5) is an extracellular glucosyltransferase that is usually produced by oral streptococci and various strains of Leuconostoc mesenteroides. Dextransucrase is induced when strain L. mesenteroides 0326 is grown on sucrose-rich media. It catalyses the transfer of d-glucosyl units from sucrose to acceptor molecules. Thus, two different products can be synthesized: α-dextran or α-oligosaccharides when efficient acceptors (like maltose) are present (Dols et al. 1997; Monchois et al. 1999). Dextran that is produced by dextransucrase of L. mesenteroides has 95% α(1 → 6) linkages in the main chains and 5% α(1 → 3) branch linkage (Brown et al. 1989). Dextran has important medical applications in the production of fine chemicals such as plasma substitutes and Sephadex. However, they can also be used in texture improvement in the food industry, e.g. in milk drinks, yoghurts and ice creams (Neubauer et al. 2003).
A gene coding for dextransucrase was isolated from L. mesenteroides NRRL B-512F and sequenced (Wilke-Douglas et al. 1989). The amino acid residues were identified, which are composed of two different functional domains: the N-terminal catalytic domain (about 900 aa) and the C-terminal domain (300–400 aa) (Monchois et al. 1999). The dextransucrase of L. mesenteroides NRRL B-512F was expressed in the presence of carbon sources other than sucrose but at low activities (Quirasco et al. 1999).
In this paper, we describe the cloning and sequencing of a dextransucrase gene (dexYG) of L. mesenteroides 0326, and the alignment of the deduced amino acid sequences with those expressed from other dextransucrase genes. Expression of the dextransucrase dexYG and its activity are also reported.
Materials and methods
Materials, bacterial strains, plasmid and medium
Restriction endonuclease, LA Taq DNA polymerase, phenol/chloroform and T4 DNA ligase were purchased from TaKaRa. E. coli DH5α was used as a host in standard cloning experiments, and was grown in Luria Bertani (LB) medium. E. coli BL21 (DE3) was used for dextransucrase expression and the strain bearing plasmid pET28a(+) were grown in LB supplemented with 50 μg kanamycin/ml.
Molecular techniques
Genomic DNA from L. mesenteroides 0326 was extracted according to the established protocols (Sambrook et al. 1989). Restriction endonucleases, LA Taq DNA polymerases were used as described by the suppliers (TaKaRa Inc.). Transformation of plasmids into E. coli, DNA purification, digestion and agarose gel electrophoresis were performed according to standard procedures (Sambrook et al. 1989). pUCm-T vector (Shanghai Sangon) was used for cloning of PCR products. DNA sequencing was carried out by Invitrogen biotechnology company (China).
Cloning of the dextransucrase gene
Based on the sequence of gene dsrD (AY017384, GenBank), two primers [sense (5′-ATTTATGCCATTTACAAAAAAGCT-3′) and antisense (5′-CTTATGCTGACACAGCATTTCC-3′] were designed to amplify the gene by PCR. The 50 μl reaction mixture contained 5 μg genomic DNA, 0.5 μM of each primers, 250 μM of each dNTPs, 5 U LA Taq DNA polymerase, 2.5 mM MgCl2 and 1 × LA Buffer (Ryu et al. 2000). The PCR amplification protocol consisted of a denaturation at 94°C for 5 min followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 47°C, for 30 s, extension at 72°C for 5 min, and a final hold for an extra 10 min at 72°C. The resulting PCR-amplified fragments were ligated into plasmid pUCm-T to generate plasmid pYGdex, and the plasmid pYGdex was transformed into the E. coli DH5α strain.
The nucleotide sequence of the dexYG gene has been submitted to the GenBank databases under accession no. DQ345760.
Construction of expression plasmid pETdex
Using the plasmid pYGdex as template, a pair of specific primers were designed as follows: Sense 5′-CCGTAGATCTTCCATGCCATTTACAGAAAAAGT-3′ with a BglII site (underlined) and Antisense 5′-CCGCTCGAGTTATGCTGACACAGCATTT-3′ with a XhoI site (underlined). The PCR product obtained was cleaved with BglII and XhoI and ligated into the vector pET28a(+) previously digested with BamHI and XhoI to construct an expression plasmid pETdex. Plasmid pETdex was then transformed into E. coli BL21 (DE3) to obtain recombinant E. coli BL21 (DE3)/pETdex.
Expression and SDS-PAGE of dextransucrase
Cells of BL21 (DE3)/pETdex were grown at 37°C in LB medium containing 50 μg kanamycin/ml, and the expression of dextransucrase was induced by adding IPTG to 1 mM when the OD600 reached 0.6. The cultivation was continued for 5 h and the cells were harvested by centrifugation at 12,000 × g for 5 min (Malten et al. 2005). Bacteria pellet was disrupted by pulse sonication in lysis buffer as crude enzyme (Funane et al. 2001).
Proteins from the lysed cells were separated by 8% (w/v) SDS-PAGE (Sambrook et al. 1989) and were stained with Coomassie Brilliant Blue. After SDS-PAGE, the gel was washed three times with 20 mM sodium acetate buffer, pH 5.4, containing 0.05 g CaCl2/l to eliminate the SDS.
Activity assay and enzymatic biotransformation
Dextransucrase activity was determined spectrophotometrically by measuring the initial rate of fructose production using the dinitrosalicylic acid method (Monchois et al. 1997). The enzymatic reaction was carried out at 30°C with magnetic stirring in 20 mM sodium acetate buffer (pH 5.4) containing 100 g sucrose/l and 0.05 g CaCl2/l. Samples were centrifuged for 8 min at 9,000 × g before measuring the absorbance. A calibration curve was obtained with a 1 g fructose/l solution. One unit of dextransucrase activity was defined as that catalyzing the formation of 0.1 mg fructose/h under the above-mentioned conditions.
Results and discussion
Sequence analysis of the cloned dexYG and comparison of dexYG with others
The nucleotide sequence of the dexYG gene was cloned and sequenced. The nucleotide sequence of the dexYG gene was 4,584 bp in length (see Supplementary Table 1) and encodes a protein of 1,527 aa (Fig. 1) with a molecular mass of 170 kDa. The highly conserved residues identified in the N-terminal domain (Neubauer et al. 2003) were also conserved in dexYG. Analysis of the dexYG protein sequence revealed that it consists of a hydrophilic core domain with a hydrophobic N-terminal region. This N-terminal region displayed the sequence characteristics of a typical signal peptide sequence for secretion. The signal peptidase cleavage site at residues between position 42 and 43 (MPFTEKVMRKKLYKVGKSWV VGGVCA FALTASFALATPSVLG-DSSVPD) was predicted using SignalP 3.0 server (http://www.cbs.dtu.dk/services/SignalP). Dextransucrase can be secreted into the culture medium of L. mesenteroides strain. However, the recombinant protein (dextransucrase) cannot be secreted expressed into the culture medium of recombinant E. coli (DE3)/pETdex since it does not have a signal sequence of the correspondent E. coli host.
The obtained results were analysed by a BLAST similarity search of the GenBank database (http://www.ncbi.nlm.nih.gov.cn/BLAST). Comparison of the deduced amino acid sequence of dexYG with the sequences of other proteins revealed similarities corresponding to some conserved regions of dextransucrases. The highest level of homology was found with DsrD (GenBank AY017384) from L. mesenteroides Lcc4 (98.8% identity). The amino acid sequence is also highly similar to DEX (GenBank LMU81374; 97.4% identity). dexYG also shows significant similarities to other dextransucrases such as DsrB (66.7% identity) (Monchois et al. 1998) and DsrC (GenBank LME250172, 65.5% identity).
Expression of dexYG in E. coli
Dextransucrase can be efficiently expressed in E. coli BL21 (DE3) by IPTG induction at 25°C. SDS-PAGE analysis clearly showed a band after 3 h induction, corresponding to a Mr 170 kDa. This value is consistent with the theoretical molecular mass (Fig. 2).
SDS-PAGE analysis of the dexYG gene product, E. coli BL21 (DE3) cells bearing pET28a (+) plasmids were cultured at 25°C in LB medium containing 0.1 M Tris/HCl buffer, pH 5.4, and 50 μg kanamycin/ml. They were induced with 1 mM IPTG. 1 ml cultures were collected at different times of incubation (2, 3 and 4 h). Proteins were extracted by boiling bacterial pellets in a denaturation buffer and analyzed by 8% (w/v) SDS-PAGE and stained with Coomassie Brilliant Blue. Lane M: molecular weight markers; Lane1: 2 h after induction; Lane2: 3 h after induction; Lane3: 4 h after induction; Lane4: 0 h after induction. Arrow indicates an induced protein band with an Mr = 170 kDa
Effect of pH on the enzymatic activity
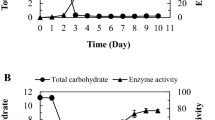
The dextransucrase activity of dexYG was detected by measuring the release of reducing sugars in the presence of sucrose (Fig. 3). The dextransucrase activity was reached 5.4 U/ml after 4 h induction at 25°C. The degradation of activity was observed in a 5 h culture after induction. The pH value was increasing from 7.4 to 8.1 during the period of induction. Data showed that the loss of activity at 5 h post induction might be due to the increase in culture pH.
Time course of pH and dextransucrase activity after induction. The dextransucrase activity was measured by the dinitrosalicylic acid method. They were induced with 1 mM IPTG, and samples were taken at various time points after induction (1, 2, 3, 4, 5 and 6 h). The enzyme reaction is described in ‘Materials and methods’. (□) pH; (●) dextransucrase activity
The effect of pH value on dextransucrase activity produced by engineered strain were studied (Fig. 4). The highest activity was reached 35.6 U/ml under the optimal culture conditions after 5 h induction in medium with pH 6.0 buffer, which was 3.5 times as that of in LB medium without pH-adjustment. The data demonstrated that the pH value was one of the main reasons which caused the degradation of enzyme activity in the later stage of induction. These results showed that dextransucrase could be efficiently heterologous expressed in E. coli and a strong dextransucrase activity had been detected and activity was shown to be pH dependent.
The effect of different pH value on dextransucrase activity produced by engineered strain. They were induced with 1 mM IPTG, and samples were taken at various time points after induction (3 ,4, 5 and 6 h). The enzyme reaction are described in ‘Materials and methods’. Different pH value in experiment: (■) pH 6.0 sodium phosphate buffer, (●) initial pH (not buffered), (Δ) pH 8.0 sodium phosphate buffer
Enzymatic biotransformation
In the biotransformation test of dextransucrase, the substrate, sucrose, was transformed to dextran and fructose within 2 h by crude enzyme and the molecular weight of dextran was greater than 68 kDa (Fig. 5). Biotransformation yield of the enzyme reached 65% by measuring production of fructose using DNS method. These data demonstrated that the dextransucrase gene dexYG of L. mesenteroides 0326 was heterologously expressed in E. coli BL21 (DE3) and was able to drive dextran synthesis. Our results on the expression of this enzyme in E. coli BL21 (DE3) fermentations may help to optimize the industrial process of dextransucrase production. These may bring new opportunities for industrial applications of dextransucrases.
HPLC analysis of the product of dextransucrase biotransformation. In the biotransformation of dextransucrase, the substrate sucrose was transformed to dextran and fructose within 2 h by crude enzyme and the molecular weight of dextran was bigger than 68 kDa. Biotransformation yield of the enzyme reached 65% by measuring production of fructose using DNS method. The enzyme reaction is described in ‘Materials and methods’. HPLC conditions: GPC column, mobile phase: ultrapure water, flow rate: 0.6 ml/min, injection volume: 10 μl, detector temperature: 40°C, column temperature 60°C
References
Brown DE, McAvoy A (1989) A pH controlled fed-batch process for dextransucrase production. J Chem Technol Biotechnol 48:405–414
Dols M, Remaud SM, Monsan PF (1997) Dextransucrase production by Leuconostoc mesenteroides NRRL B-1299. Comparison with Leuconostoc mesenteroides NRRL B-512F. Enzyme Microbiol Technol 20:523–530
Funane K, Ishii T, Matsushita M, Hori K, Mizuno K, Takahara H, Kitamura Y, Kobayashi M (2001) Water-soluble and water-insoluble glucans produced by Escherichia coli recombinant dextransucrases from Leuconostoc mesenteroides NRRL B-512F. Carbohydr Res 334:19–25
Malten M, Hollmann R, Deckwer WD, Jahn D (2005) Production and secretion of recombinant Leuconostoc mesenteroides dextransucrase DsrS in Bacillus megaterium. Biotechnol Bioeng 89:206–218
Monchois V, Remaud-Simeon M, Russell RRB, Monsan P, Willemot RM (1997) Characterization of Leuconostoc mesenteroides NRRL B-512F dextransucrase (DSR-S) and identification of amino-acid residues playing a key role in enzyme activity. Appl Microbiol Biotechnol 48:465–472
Monchois V, Remaud-Simeon M, Monsan P, Willemot RM (1998) Cloning and sequencing of a gene coding for an extracellular dextransucrase (dsrB) from Leuconostoc mesenteroides NRRL B-1299 synthesizing only a (1–6) glucan. FEMS Microbiol Lett 159:307–315
Monchois V, Willemot RM, Monsan P (1999) Glucansucrases: mechanism of action and structure–function relationships. FEMS Microbiol Lett 23:131–151
Neubauer H, Bauche A, Mollet B (2003) Molecular characterization and expression analysis of the dextransucrase DsrD of Leuconostoc mesenteroides Lcc4 in homologous and heterologous Lactococcus lactis cultures. Microbiology 149:973–982
Quirasco M, Lopez-Munguia A, Remaud-Simeon M, Monsan P, Farres A (1999) Induction and transcription studies of the dextransucrase gene in Leuconostoc mesenteroides NRRL B-512F. Appl Environ Microbiol 65:5504–5509
Ryu HJ, Kim D, Kim DW, Moon YY, Robyt JF (2000) Cloning of a dextransucrase gene (fmcmds) from a constitutive dextransucrase hyper-producing Leuconostoc mesenteroides B-512FMCM developed using VUV. Biotechnol Lett 22:421–425
Sambrook J, Fritsch EF, Maniatis T (1989). Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor, New York
Wilke-Douglas M, Perchorowicz JT, Houch CM, Thomas BR (1989) Methods and compositions for altering physical characteristics of fruit and fruit products. PCT Patent WO89/12386
Acknowledgements
This research was supported by Science and Technology Commission of Shanghai Municipality (Grant No. 07dz22002 & 04DZ05902) and Key Programs of Anhui Province College Science Research (Grant No. KJ2008A067).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, H., Hu, Y., Zhu, C. et al. Cloning, sequencing and expression of a dextransucrase gene (dexYG) from Leuconostoc mesenteroides . Biotechnol Lett 30, 1441–1446 (2008). https://doi.org/10.1007/s10529-008-9711-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-008-9711-8