Abstract
To develop an integrated process of CO2-fixation and H2 photoproduction by marine green microalga Platymonas subcordiformis, the impact of algal cells grown in CO2-supplemented air bubble column bioreactor was investigated on H2 photoproduction regulated by carbonylcyanide m-chlorophenylhrazone. Highest cell growth (3.85 × 106 cells ml−1), starch content (0.25 ± 0.08 mg per 106 cells) and hydrogen production (50 ± 3 ml l−1) were achieved at 3% CO2-supplemented culture, which are respectively 1.4, 2.1, 1.5-fold of the air-supplemented culture. Improved H2 production correlated well with the increase in starch accumulation. In this process, the algal cells have been recycled for stable H2 production of 40–50 ml l−1 over five cycles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Photobiological H2 production by unicellular green algae has attracted great attention due to their ability to combine CO2 fixation with H2 production directly from water and solar energy (Gaffron and Rubin 1942; Ghirardi et al. 2000). H2 photoproduction by microalgae has been documented among the genus Chlorococcales and Volvocales (Chlorophyceae) (Happe et al. 2002), with some algae tolerant to extremely high-CO2 concentration (≤50%) and even in exhaust flue gas (Iwasaki et al. 1998). Therefore, CO2 mitigation and concurrent H2 production by microalgae could become an important technology for the reduction of climate impact of greenhouse gas emissions and renewable energy production, with numerous other advantages.
The freshwater green alga, Chlamydomonas reinhardtii, is a well-established model strain that produces H2 photobiologically under sulfur deprivation in a two-stage system (Melis et al. 2000). Considerable attempts have been made to optimize the process in C. reinhardtii under different growth conditions (Ghirardi et al. 2000; Kosourov et al. 2007). CO2 is required as substrate for photosynthetic production of intracellular starch. Starch is then required to maintain anaerobic conditions essential for H2 production by mitochondrial respiration to deplete O2. In addition, starch degradation also provides some electrons for H2 production (Kosourov et al. 2002, 2007). As reported by Kruse et al. (2005), engineered green algal cells with higher starch accumulation exhibit a significant increase in the rate and duration of H2 production.
In our earlier work, a marine green alga, P. subcordiformis, was shown to photobiologically evolve H2 under the regulation of carbonylcyanide m-chlorophenylhrazone (CCCP) (Guan et al. 2004a, b; Ran et al. 2006). The yield and the duration of H2 production were lower than those in C. reinhardtii under sulfur deprivation. Different regulation characteristics have been observed in how the two algal species respond to sulfur-deprivation (Guan et al. 2004a; Ran et al. 2006). While Platymonas subcordiformis did not respond to sulfur-deprivation, it produced significant amounts of H2 under the regulation of CCCP. The addition of CCCP and sulfur-deprivation achieved a similar effect in terms of decreasing PSII activity and allowing the establishment of anaerobiosis essential for H2 production. However the intracellular starch accumulation limits H2 yield due to the fact that anaerobiosis can only be maintained until starch is depleted by mitochondria respiration (Kruse et al. 2005; Ran et al. 2006).
To further improve H2 production, this paper investigates two new process strategies of CO2-supplementation and the use of an air bubble column photobioreactor to increase both cell growth and starch accumulation of P. subcordiformis, eventually leading to improved H2 production.
Materials and methods
Growth of Platymonas subcordiformis
P. subcordiformis, wild-type, was grown photoautotrophically in a 600 ml bubble column bioreactor system (50 mm diam, 400 mm ht) (Fig. 1) at 25°C in an autoclaved natural seawater medium, supplemented with micronutrients with initial pH 8.0 as described earlier (Ran et al. 2006). Compressed air and CO2 were mixed and metered through calibrated flow meters, and sterilized using 0.22 μm membrane filters before entering the system. The average aeration rate was 0.2 vvm. The bottom of the bioreactor was filled with a porous quartz sieve (10 mm diam), which dispersed the airstream. The cultures were illuminated from two sides with cool-white fluorescent lamps which provided an average light intensity of 50 μE m−2 s−1 under 14 h: 10 h light: dark cycle. CO2 was added up to 15% (v/v) in air. The CO2 was supplemented only during illumination. Cell density was determined by cell counting using a hemacytometer.
Schematic diagram of the bubble column bioreactor system used for the growth of P. subcordiformis cultures under photoautotrophic conditions. (1) CO2 bottle, (2) air compress pump, (3) manual valve, (4) gas flowmeter, (5) bubble column bioreactor, (6) cool-white fluorescent light, and (7) porous sieve
Dark anaerobic incubation and photobiological H2 production
For H2 production, 7-day old cells from the bioreactor were harvested by centrifugation at 250 g for 2 min. Cells were concentrated to 6 × 106 cells ml−1 in the spent medium at pH 8.0. The concentrated cultures were transferred into a 500 ml cylindrical glass bioreactor system for H2 production as described in Ran et al. (2006). Briefly, the sealed cultures were subjected to the dark anaerobic induction of hydrogenase at 25°C for 12 h after 10 min continuous flushing with N2 (99.9% purity) through the culture to remove O2 from the system. A total of 15 μM CCCP was then added to the cultures and further incubated in darkness for 20 min. The cultures were then initiated for photobiological H2 production under continuous illumination by cool-white fluorescent light (80 μE m−2 s−1).
Gas collection, H2 concentration and volume measurements
Gases produced by the culture were accumulated in the burette by displacing an equal volume of water. H2 was analyzed by GC equipped with a thermal conductivity detector and a 5 Å molecular sieve column with Ar as carrier gas. The volume of H2 was calculated by its concentration multiplying the total gas volumes collected.
PS П photochemical activity measurement
Chlorophyll a fluorescence yields were measured with a Water-Pam fluorometer (Walz, Germany) according to the manufacture’s instructions. The steady state fluorescence level (Ft) was measured under the actinic light (150 μE m−2 s−1) prior to a saturating light pulse. The maximum fluorescence level (Fm′) was induced by a saturating actinic light pulse (0.8 s, 4000 μE m−2 s−1). The PS II photochemical activity of the cells was calculated as Yield = ΔF/Fm′ = (Fm′−Ft)/Fm′.
In vitro hydrogenase activity measurement
Aliquots of P. subcordiformis were taken anaerobically from the bioreactor during the course of illumination. Gas-tight sample tubes containing 2 ml 50 mM phosphate buffer pH 7.0, 25 mM sodium diothinite, 5 mM Methyl Viologen and 500 μl algae culture, were incubated at 37°C for 20 min (Kosourov et al. 2003). H2 evolution was quantified by GC.
Other analytical procedures
Protein and starch contents inside the cells were determined according to the methods of Lowry and Klein and Betz (1978), respectively.
Stability of H2 photoproduction from the recycled culture after rejuvenation
To investigate if the cultures after H2 production can be recycled for repeated H2 production, the cells were harvested, washed three times and re-suspended in the same medium at 106 cells ml−1and rejuvenated for 10–15 days supplemented with 3% CO2 (v/v) in the air bubble column bioreactor, then transferred for H2 production as described in Ran et al. (2006).
Results and discussion
Photoautotrophic growth of P. subcordiformis under different CO2 concentrations
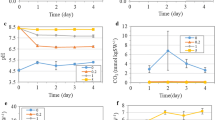
Figure 2 shows the kinetics of cell growth, pH and PS Π photochemical activity (ΔF/Fm′) when grown with air and different concentrations of CO2-supplemented air. The overall specific growth rate of P. subcordiformis was 0.1, 0.12, 0.13, 0.126, 0.1 and 0.08 day−1 in air, 1%, 3%, 5%, 10% CO2 and 15% CO2-grown cells, respectively (Fig. 2a). The maximum cell concentration was attained when 3% CO2 was used. This clearly indicated that air (containing 0.03% CO2) is deficient for optimal growth (Guan et al. 2004a; Ran et al. 2006) and that CO2 supplementation is essential for improved algal cell growth under photoautotrophic conditions.
Kinetics in cell density (a), pH (b) and PS Π photochemical activity (ΔF/Fm′) (c) during the cultures of P. subcordiformis under photoautotrophic conditions in air (■), 1% CO2 (□), 3% CO2 (●), 5% CO2 (○), 10% CO2 (▲) and 15% CO2-grown cells (Δ). Cells were grown in air bubble column bioreactor at the light intensity of 50 μE m−2 s−1 under 14 h: 10 h light: dark cycle. Data indicate mean ± SD (n = 3)
As shown in Fig. 2b, the pH in the air-grown culture increased from an initial pH 8.0 to a maximum pH 9.6 on day 4. The initial increase in pH is due to the photosynthetic consumption of dissolved CO2 and then the pH value remained constant. When the algal cells were grown under CO2-supplemented air, the pH continually decreased. This decrease in pH was expected because CO2 supplementation buffered the medium as a result of the CO2/HCO −3 balance. The decrease in pH had a positive correlation with the supplementation of CO2 concentrations. Previous studies showed that carbonic anhydrase (CA) and a CO2-concentrating mechanism (CCM) played important roles for efficient utilization of dissolved inorganic carbon under CO2-limited conditions (Saton et al. 2001). When algal cells were exposed to high concentrations of CO2 (>10%), intracellular CA caused intracellular acidification and hence inhibition of photosynthetic carbon fixation, which explained the growth inhibition at high CO2 concentrations (>10%).
To estimate the actual photochemical activity of PS Π (ΔF/Fm′), the chlorophyll a fluorescence of cultures in the bubble column bioreactors was measured. Figure 2c shows that PS Π activity remained nearly constant at about 0.6 in air-grown cells. However, PS Π activity started to decline with the increase of CO2 concentration from day 4. The decrease of PS Π activity was correlated with the increase in CO2 concentration. Higher CO2 concentration probably suppressed the photosynthetic electron transport, leading to greater QA reductions owing to acidification caused by intracellular CA in chloroplasts (Saton et al. 2001).
H2 yield, PS Π activity and in vitro hydrogenase activity during CCCP-regulated H2 photoproduction
The H2 yield, PS Π activity and in vitro hydrogenase activity of cells grown under different CO2 concentrations were compared (Fig. 3). H2 production was detected soon after the beginning of illumination and its yield was higher in CO2-grown cells when compared to that of air-grown cells. H2 photoproduction was enhanced by ∼60% for 3% CO2-grown cells when compared to air-grown cells, with the highest rate of H2 production 3.2 ± 0.12 μmol H2 mg−1Chl h−1. The results were in agreement with Yoon et al. (2002) who reported that CO2 injection in the cell growth phase increased not only the cell concentration but also the H2 production per g of Anabaena variabilis.
Comparison of hydrogen yield (a), PS Π photochemical activity (ΔF/Fm′) (b) and in vitro hydrogenase activity (c) in air (■), 1% CO2 (□), 3% CO2 (•), 5% CO2 (○) and 10% CO2-grown cells (▲) during CCCP-regulated H2 photoproduction phase under 80 μE m−2 s−1 of light. Cells from air bubble column bioreactor were transferred to the cylindrical glass bioreactor for 12 h dark anaerobiosis and 15 μM CCCP-regulated H2 photoproduction of P. subcordiformis under 80 μE m−2 s−1 of light. Data indicate mean ± SD (n = 3)
To examine the relationship between the PS Π activity and H2 production, PS Π activity of cell cultures under different CO2 concentrations was measured during H2 photoproduction stage. As shown in Fig. 3b, PS Π activity was lower in CO2-grown cells when compared to air-grown cells at the onset of H2-production. PS Π activity decreased rapidly within 4 h and declined slowly thereafter. Antal et al. (2001) suggested that the decline in ΔF/Fm′ reflected the PQ pool over-reduction under stress conditions such as sulfur-deprivation. In addition, the drop in PS Π activity could indicate state transition process which regulated the light activation of PS I and PS II, by adjusting their respective light harvesting antenna sizes under anaerobic conditions. This result suggested that CCCP down-regulated PS II activity of the sealed P. subcordiformis cultures and anaerobiosis maintained during illumination, thereby simultaneously hydrogenase induced, thus contributing to H2 photoproduction (Ran et al. 2006).
Hydrogenase activity was critical as an electron valve for sustained H2 production (Happe et al. 2002). Figure 3c shows that the hydrogenase activity in CO2-grown cells was slightly higher when compared to that of air-grown cells at the end of 12 h dark anaerobic induction, and then declined gradually during the H2-production stage. Kosourov et al. (2003) proposed that the decline in the hydrogenase activity when a pH shift either to the alkaline or acidic side was due to the direct effect of pH on the catalytic function of the enzyme. Under anoxic conditions, green algae could switch their metabolism towards fermentation and the formation of mixed acids resulted in the sharp decrease in pH (Hemschemeier and Happe 2005). In the same manner, it could be explained that the pH shift exerted an influence on the gradual decline in the hydrogenase activity of P. subcordiformis cells during illumination whereas the optimum physiological pH of the cultures was 7.2–8.2.
Changes in pH, protein and starch during dark anaerobiosis and H2 photoproduction
As shown in Table 1, at the end of 12 h dark anaerobiosis the extracellular ΔpH of all cultures ranged from −1.96 ± 0.11 (air-grown culture) to −2.92 ± 0.13 (10% CO2-grown culture). The sharp decrease in pH may attribute to the metabolism switch of algae cells towards fermentation and the formation of mixed acids under dark anoxic conditions (Hemschemeier and Happe 2005). At the start of illumination for H2 production, the pH in all cultures was readjusted to 7.5. However, at the end of 24 h CCCP-regulated H2 photoproduction, a remarkable difference was observed in the change of pH between the air-grown cells and the CO2-grown cells. The extracellular ΔpH of the air-grown culture was −1.07 ± 0.08 and increased with the supplementation of higher CO2 concentrations, reaching −1.79 ± 0.06 for 10% CO2-grown culture. The fermentative metabolic switch from dark fermentation to light fermentation resulted in the continuous pH decline. These data were in agreement with an earlier observation of Melis et al. (2000) who showed that green alga C. reinhardtii could switch to fermentative products.
There are two major pathways for H2 production in Chlamydomonas (Kosourov et al. 2002, 2007), involving both reductants generated by water oxidation at PSII and reductants released upon starch and protein degradation. Higher H2 yield could be achieved with increased starch accumulation as a result of fixing more CO2 into starch by photosynthesis in photoautotrophic cultures. As shown in Tables 2 and 3, protein and starch content was higher in CO2-supplemented-grown cells when compared to air-grown cells at the start of dark anaerobiosis. The 3% CO2-grown cells have the highest protein and starch contents per cell that are 1.2- and 2.1-fold compared to the control. Cellular protein and starch contents declined in all cultures during dark anaerobic phase and H2-production phase. Protein degradation and starch catabolism were required for S-deprived C. reinhardtii H2 production (Ghirardi et al. 2000; Melis et al. 2000). Likewise, in our experiment the declines in the total cellular protein and starch content in all cultures were concomitant with the H2-production process. In addition, our results were also consistent with the findings of Kosourov and co-workers (2007) who reported that under photoautotrophic conditions H2 production could be increased by optimizing the conditions for higher starch accumulation. Higher consumption of starch and protein in CO2-supplemented-grown cells was likely responsible for the enhanced yield of H2 production (Tables 2 and 3).
Repeated H2 photoproduction from recycled cultures
Cultures of P. subcordiformis could be rejuvenated by CCCP removal and replenishing fresh micronutrients medium for repeated H2 production to establish cycles between CO2-fixation photosynthesis stage and CCCP-regulated H2 photoproduction stage for five times (Fig. 4). The average H2 production volumes of the five experiments were 46.2 ± 2.8 ml H2 l−1. The results demonstrated that the effect of CCCP on the H2 photoprodution from marine green alga P. subcordiformis was reversible and reproducible, which could lead to the development of a marine green algal continuous H2-production system.
Repeated H2 photoproduction from recycled P. subcordiformis cultures. At the end of 24 h, H2 photoproduction in the first cycle, the cultures were harvested and washed three times with the fresh replete micronutrients natural seawater and re-suspended in the same medium to 106 cells ml−1 and rejuvenated for 10–15 days supplemented with 3% CO2 (v/v). Subsequently, the sealed cultures were subjected to dark anaerobic phase and CCCP-regulated H2 photoproduction phase. The cultures were recycled between CO2-fixation photosynthesis stage and CCCP-regulated H2 photoproduction stage for five times. Data indicate mean ± SD (n = 3)
In conclusion, marine green alga P. subcordiformis can efficiently convert CO2 into intracellular starch through photosynthesis and improve H2 photoproduction through increased accumulation of intracellular starch under CO2 supplementation in an air bubble column photobioreactor. The concentrations of CO2 supplemented showed significant impact on the kinetic parameters of photosynthesis and H2 photoproduction. The combination of CO2 fixation and enhanced H2 photoproduction by marine green alga demonstrates great potential to a significant decrease in the costs associated with CO2 mitigation and H2 production. However, further research efforts are required to optimize growth conditions for high starch accumulation that might be manipulated to significantly improve the yield and duration of H2 production for any large-scale trial.
References
Antal TK, Krendeleva TE, Laurinavichene TV, Makarova VV, Tsygankov AA, Seibert M, Rubin AB (2001) The relationship between the photosystem 2 activity and hydrogen production in sulfur deprived Chlamydomonas reinhardtii cells. Dokl Biochem Biophys 381:371–374
Gaffron H, Rubin J (1942) Fermentative and photochemical production of hydrogen in algae. J Gen Physiol 26:219–240
Ghirardi ML, Zhang L, Lee JW, Flynn T, Seibert M, Greenbaum E, Melis A (2000) Microalgae: a green source of renewable H2. Trends Biotechnol 18:506–511
Guan YF, Deng MC, Yu XJ, Zhang W (2004a) Significant enhancement of photobiological hydrogen evolution by carbonylcyanide m-chlorophenyl hydrazone in the marine green alga Platymonas subcordiformis. Biotechnol Lett 26:1031–1035
Guan YF, Deng MC, Yu XJ, Zhang W (2004b) Two-stage photo-biological production of hydrogen by marine green alga Platymonas subcordiformis. Biochem Eng J 19:69–73
Happe T, Hemschemeier A, Winkler M, Kaminski A (2002) Hydrogenases in green algae: do they save the algae’s life and solve our energy problems? Trends Plant Sci 7:246–250
Hemschemeier A, Happe T (2005) The exceptional photofermentative hydrogen metabolism of the green alga Chlamydomonas reinhardtii. Biochem Soc Trans 33:39–41
Iwasaki I, Hu Q, Kurano N, Miyachi S (1998) Effect of extremely high-CO2 stress on energy distribution between photosystem I and photosystem II in a ‘high-CO2’ tolerant green alga, Chlorococcum littorale and the intolerant green alga Stichococcus bacillaris. J Photoch Photobio B 44:184–190
Klein U, Betz A (1978) Fermentative metabolism of hydrogen-evolving Chlamydomonas moewusii. Plant Physiol 61:953–956
Kosourov S, Tsygankov A, Seibert M, Ghirardi ML (2002) Sustained hydrogen photoproduction by Chlamydomonas reinhardtii: effects of culture parameters. Biotechnol Bioeng 78:731–740
Kosourov S, Seibert M, Ghirardi ML (2003) Effects of extracellular pH on the metabolic pathways in sulfur-deprived H2−producing Chlamydomonas reinhardtii cultures. Plant Cell Physiol 44:146–155
Kosourov S, Patrusheva E, Ghirardi ML, Seibert M, Tsygankov A (2007) A comparison of hydrogen photoproduction by sulfur-deprived Chlamydomonas reinhardtii under different growth condition. J Biotech 128:776–787
Kruse O, Rupprecht J, Bader KP, Thomas-Hall S, Schenk PM, Finazzi G, Hankamer B (2005) Improved photobiological H2 production in engineered green algal cells. J Biol Chem 280:34170–34177
Melis A, Zhang L, Forestier M, Ghirardi ML, Seibert M (2000) Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol 122:127–136
Ran CQ, Yu XJ, Jin MF, Zhang W (2006) Role of carbonyl cyanide m-chlorophenylhydrazone in enhancing photobiological hydrogen production by marine green alga Platymonas subcordiformis. Biotechnol Prog 22:438–443
Saton A, Kurano N, Miyachi S (2001) Inhibition of photosynthesis by intracellular carbonic anhydrase in microalgae under excess concentrations of CO2. Photosynth Res 68:215–224
Yoon JH, Sim SJ, Kim MS, Park TH (2002) High cell density culture of Anabaena variabilis using repeated injections of carbon dioxide for the production of hydrogen. Int J Hydrogen Energy 27:1265–1270
Acknowledgements
The financial supports are provided by National Basic R&D “973 Program’’ (2003CB214506), National High Technology R&D ‘‘863 Program’’ (2006AA05Z106), and “Innovation Fund” (KSCX2-YW-G-002) by the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, Z., Chen, Z., Zhang, W. et al. Improved hydrogen photoproduction regulated by carbonylcyanide m-chlorophenylhrazone from marine green alga Platymonas subcordiformis grown in CO2-supplemented air bubble column bioreactor. Biotechnol Lett 30, 877–883 (2008). https://doi.org/10.1007/s10529-008-9637-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-008-9637-1