Abstract
The EntD-like phosphopantetheinyl transferase (PPTase) gene, cloned from the eicosapentaenoic acid-producing bacterium Photobacterium profundum strain SS9, has an ORF of 690 bp encoding a 230-amino acid protein. When this PPTase gene was expressed in Escherichia coli with pfaA, pfaB, pfaC and pfaD derived from Moritella marina MP-1, which were four of five essential genes for biosynthesis of docosahexaenoic acid (DHA), the DHA production of the recombinant was 2% (w/w) of total fatty acids. This is the first report showing that the EntD-like PPTase is involved in producing n-3 polyunsaturated fatty acids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
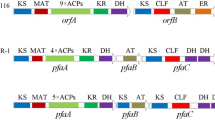
Long-chain n-3 polyunsaturated fatty acids (n-3 PUFAs), such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are synthesized de novo in bacteria via polyketide biosynthesis (Metz et al. 2001; Orikasa et al. 2006a, b, c). The genes (pfaA, pfaB, pfaC, pfaD, and pfaE) involved in biosynthesis of EPA or DHA have been cloned from EPA-producing or DHA-producing bacteria, respectively (Allen and Bartlett 2002; Orikasa et al. 2004; Tanaka et al. 1999). The gene structures and domain structures of these five genes essential for biosynthesis of EPA or DHA were recently reviewed (Okuyama et al. 2007).
Photobacterium profundum SS9 is an EPA-producing bacterium that was isolated from deep seawater (Allen and Bartlett 2000). Although pfaA, pfaB, pfaC, and pfaD genes were cloned from P. profundum SS9 (Allen and Bartlett 2002), the gene encoding phosphopantheteinyl transferase (PPTase) corresponding to pfaE was not obtained. According to the P. profundum SS9 genome database (Vezzi et al. 2005, http://getentry.ddbj.nig.ac.jp/top-j.html), this bacterium has two putative PPTase genes. One (accession number CAG21401) is considered from its size (378 bp) to be an AcpS-type PPTase, and to be involved in the de novo biosynthesis of fatty acids, and the other (accession number CAG23685) is a 690-bp putative PPTase, with a primary structure similar to that of Sfp-type PPTases.
According to Orikasa et al. (2006b) Sfp-type PPTases can be divided into two groups. The first group includes PPTases mainly used for n-3 PUFA synthesis, while the other group includes PPTases principally involved in polyketide and/or nonribosomal peptide synthesis. The SS9 putative Sfp-type PPTase and EntD of Escherichia coli, which is responsible for the synthesis of siderophore enterobactin (Hantash et al. 1997), are included in the latter group (Orikasa et al. 2006b). The SS9 putative Sfp-type PPTase is regarded as EntD (Vezzi et al. 2005). The two groups have P2 (GxDxE) and P3 (KExxxKx) domains (where x is a nonconserved amino acid) in common. The PPTases necessary for the biosynthesis of n-3 PUFA have highly conserved P0 (L/VRxL/VLS), P1a (KxKP), and P1b (FNxSH) domains at their N-terminal region. However, in PPTases involved in the biosynthesis of polyketides and/or nonribosomal peptides, these domains are replaced with 1A (KRxxEx), P1a′ (xRxP), and P1b′ (GSIxH) domains, respectively (Orikasa et al. 2006b).
In this study, the putative Sfp-type EntD-like PPTase gene (CAG23685) was cloned from the P. profundum SS9 genome and coexpressed with pfaA–D genes from DHA-producing Moritella marina MP-1.
Materials and methods
Bacterial strains and culture conditions
Bacterial strains and vectors used in this study are listed in Table 1. Escherichia coli recombinant cells were cultivated by shaking in Luria Bertani (LB) medium supplemented with indicated antibiotics at 37°C for 16 h. A portion of the E. coli DH5α cells carrying pET21::pfaE (see below) and pDHA3 (Orikasa et al. 2006a, c) that were precultured at 37°C was transferred to fresh LB medium and then cultivated at 15°C for 48 h for DHA production.
PCR and plasmid construction
Chromosomal DNA of SS9 (Allen and Bartlett 2002) was kindly provided by Dr. D. H. Bartlett (Center for Marine Biotechnology and Biomedicine, Scripps Institution of Oceanography, San Diego, La Jolla, California). To clone the full length of the PPTase gene (designated pfaE) of SS9, PCR was carried out using one set of oligonucleotide primers: forward (5′-GAAACCTCAATAATACATATGAA-3′) including an NdeI site (underlined) and reverse (5′-TCATTATGAATTCATTCAAC-3′) including an EcoRI site (underlined) and SS9 genome DNA as template. The resulting DNA fragment including the 690 bp ORF was digested with NdeI and EcoRI, cloned into pCR2.1-TOPO (pCR2.1-TOPO::pfaE) and used for transformation of E. coli DH5α. The pCR2.1-TOPO::pfaE was treated with NdeI and EcoRI and then the resulting insert DNA was cloned into NdeI–EcoRI-digested pET21a (pET21a::pfaE). The pfaE gene sequence was determined by the dideoxy-chain termination method as described previously (Orikasa et al. 2006a).
SDS-PAGE of proteins and GC and GC/MS analysis of fatty acids
Escherichia coli BL21 (DE3) carrying either pET21a::pfaE or pET21a was cultivated at 37°C for 16 h or at 15°C for 96 h in LB medium containing ampicillin at 50 μg ml−1. Expression of the pfaE gene was induced by the addition of isopropyl β-d-thiogalactoside (IPTG) at 0.3 mM. The proteins produced by the recombinant bacteria were analyzed by SDS-PAGE, as described previously (Orikasa et al. 2006a, c).
Cells were directly methanolyzed and the resulting fatty acid methyl esters were analyzed by gas-liquid chromatography (GC) and gas chromatography/mass spectrometry (GC/MS), as described previously (Orikasa et al. 2006a, c).
Results and discussion
Cloning and characterization of pfaE from P. profundum SS9
The PPTase gene of 690 pb was cloned as pCR2.1-TOPO::pfaE, and then integrated into pET21a (pET21a::pfaE). The pfaE of SS9 encodes a deduced protein consisting of 230 amino acids. Its calculated molecular mass of 25.5 kDa is slightly less than that of other Sfp-type PPTases involved in biosynthesis of n-3 PUFA (Orikasa et al. 2006a) and close to that (23.6 kDa) of EntD of E. coli.
Expression of pfaE in E. coli
In SDS-PAGE analysis, the recombinant E. coli BL21(DE3) harboring pET21a::pfaE, which was grown at 37°C and then treated with IPTG at 0.3 mM, showed an intense band of 25 kDa (not shown). No band corresponding to a PPTase was observed from recombinant cells that were not treated with IPTG or from cells that carried an empty vector (not shown, see Orikasa et al. 2006c).
pET21a::pfaE was able to complement pDHA3, a vector carrying an insert DNA derived from the genome of MP-1 that included pfaA–D but no PPTase gene (pfaE) (Orikasa et al. 2006a, c). GC-based analysis of the total fatty acid methyl esters of the recombinant cells carrying pDHA3 and pET21a::pfaE grown at 15°C showed an unknown peak with a retention time of 31 min (Fig. 1a), which was the same as that of known DHA (data not shown). The GC/MS profile of this unknown component was the same as that of known DHA (not shown) and the [M–H]+ and base peak ions at m/z 341 and at m/z 79, respectively, were evident (Fig. 1b). Analysis of the fragmentation profile with a program from the National Institute of Standard and Technology databases (http://www.nist.gov./srd/nist1a/htm) indicated that the profile of the unknown component was closest to that of DHA. From these results, we conclude that this component is DHA, making up 2.0% (w/w) of total fatty acids, and that this pfaE is a PPTase gene involved in the biosynthesis of DHA. It is predicted that this PPTase is involved in the in situ biosynthesis of EPA in SS9 cells.
(a) Gas chromatogram of total fatty acid methyl esters prepared from E. coli DH5α carrying pET21a::pfaE and pDHA3. Peaks a and b are those of cis-vaccenic acid and 3-hydroxy tetradecanoic acid, respectively. Heneicosanoic acid (peak c) was used as internal standard. (b) The mass spectrum of the unknown peak with a retention time of 31 min shown in (A). E. coli DH5α cells carrying pET21::pfaE and pDHA3 were cultivated at 15°C for 48 h in LB medium containing ampicillin and chloramphenicol at 50 μg ml−1 and 30 μg ml−1, respectively
Previously, all pfaE genes involved in the biosynthesis of n-3 PUFA were considered Spf-type PPTases with P0, P1a, and P1b domains at their N-terminal region (see Orikasa et al. 2006b; Okuyama et al. 2007). However, the present study shows that the SS9 EntD-like PPTase (pfaE), which is included in the group of Sfp-type PPTases possessing 1A, P1a′, and P1b′ domains, can also be involved in the biosynthesis of n-3 PUFA. This is, to our knowledge, is the first report showing that an EntD-like PPTase is responsible for the biosynthesis of n-3 PUFA.
Although E. coli DH5α should have had its own EntD gene in its genome, the recombinant cells transformed with pDHA3 that carried only pfaA–pfaD from M. marina MP-1 or those with pEPAΔ2 carrying only pfaA–pfaD from EPA-producing Shewanella pneumatophori SCRC-2738 produced neither DHA nor EPA (Orikasa et al. 2006a,c). Considering that any pfaE cloned to date was coexpressed in high copy number plasmids (Orikasa et al. 2004, 2006a, c), relatively high amounts of E. coli EntD protein (PPTase) compared with that of PfaA–D proteins may be needed for recombinant production of EPA or DHA in E. coli.
References
Allen EE, Bartlett DH (2002) Structure and regulation of the omega-3 polyunsaturated fatty acid synthase genes from the deep-sea bacterium Photobacterium profundum strain SS9. Microbiology 148:1903–1913
Metz JG, Roessler P, Facciotti D, Levering C, Dittrich F, Lassner M, Valentine R, Lardizabal K, Domergue F, Yamada A, Yazawa K, Knauf V, Browse J (2001) Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 293:290–293
Okuyama H, Orikasa Y, Nishida T, Morita N (2007) Bacterial genes responsible for the biosynthesis of eicosapentaenoic and docosahexaenoic acids and their heterologous expression. Appl Environ Microbiol 73:665–670
Orikasa Y, Yamada A, Yu R, Ito Y, Nishida T, Yumoto I, Watanabe K, Okuyama H (2004) Characterization of the eicosapentaenoic acid biosynthesis gene cluster from Shewanella sp. strain SCRC-2738. Cell Mol Biol 50:625–630
Orikasa Y, Nishida T, Hase A, Watanabe K, Morita N, Okuyama H (2006a) A phosphopantetheinyl transferase gene responsible for biosynthesis of n-3 polyunsaturated fatty acids from Moritella marina strain MP-1. FEBS Lett 580:4423–4429
Orikasa Y, Nishida T, Watanabe K, Morita N, Okuyama H (2006b) Phosphopantetheinyl transferase genes essential for biosynthesis of polyunsaturated fatty acids and their domain structures and compatibility. In: Benning C, Ohlrogge J (eds) Advances in plant lipid research: Proceedings of the 17th International Symposium on Plant Lipids, East Lansing, Michigan, 16–21 July 2006, Michigan State University Press, East Lansing, MI, pp 169–173
Orikasa Y, Nishida N, Yamada A, Yu R, Watanabe K, Hase A, Morita N, Okuyama H (2006c) Recombinant production of docosahexaenoic acid in a mode of polyketide biosynthesis in Escherichia coli. Biotechnol Lett 28:1841–1847
Tanaka M, Ueno A, Kawasaki K, Yumoto I, Ohgiya S, Hoshino T, Ishizaki K, Okuyama H, Morita N (1999) Isolation of clustered genes that are notably homologous to the eicosapentaenoic acid biosynthesis gene cluster from the docosahexaenoic acid-producing bacterium Vibrio marinus strain MP-1. Biotechnol Lett 21:939–945
Vezzi A, Campanaro S, D’Angelo M, Simonato F, Vitulo N, Lauro FM, Cestaro A, Malacrida G, Simionati B, Cannata N, Romualdi C, Bartlett DH, Valle G (2005) Life at depth: Photobacterium profundum genome sequence and expression analysis. Science 307:1459–1461
Author information
Authors and Affiliations
Corresponding author
Additional information
Shinji Sugihara and Yoshitake Orikasa contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sugihara, S., Orikasa, Y. & Okuyama, H. An EntD-like phosphopantetheinyl transferase gene from Photobacterium profundum SS9 complements pfa genes of Moritella marina strain MP-1 involved in biosynthesis of docosahexaenoic acid. Biotechnol Lett 30, 411–414 (2008). https://doi.org/10.1007/s10529-007-9579-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-007-9579-z