Abstract
A reliable quantification of intracellular concentrations of intermediates in microorganisms depends on a proper sampling procedure and the subsequent fast inactivation of metabolism via quenching. A single device integrating both operations was developed and simultaneously the quenching procedure on cells was assessed too, without finding negative effects on viability or metabolite leakage. Moreover, supported by an experimental design, the influences of process parameters in its dynamic operation were characterized and optimized. The novel in-situ rapid sampling and quenching apparatus can be employed on any laboratory glass fermenters accessible from the top of the bioreactor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A typical sampling procedure for intracellular metabolite analysis comprises the following steps: the harvest of a representative sample from the bioreactor and fast inactivation of metabolism in the sample. Such a rapid sampling and quenching step is crucial to obtain realistic in-vivo metabolite concentrations since intracellular conversion rates are often high (in the range of 1 mM s−1) while intracellular concentrations are low (in the range of 1 mM).
A fast metabolic arrest is commonly achieved by a rapid change in temperature, accomplished by mixing the cell sample with an appropriate quenching solution. Some conventional quenching solutions are cold methanol (De Koning and van Dam 1992; Schäfer et al. 1999; Lange et al. 2001) or liquid N2 (Hajjaj et al. 1998; Chassagnole et al. 2002). The advantages of a separate quenching step before metabolite extraction are the separation of cells from supernatant to gain higher metabolite concentrations for a more accurate analysis and the possibility to distinguish between intra- and extra-cellular metabolites. The direct spraying of cell samples in perchloric acid inactivates metabolism and extracts metabolites simultaneously (Theobald et al. 1993; Larsson and Törnkvist 1996; Weuster-Botz 1997).
Since the early 1990s several systems have been established to enable a rapid mixing of sample with the quenching solution (Theobald et al. 1993; Larsson and Törnkvist 1996; Weuster-Botz 1997; Schäfer et al. 1999; Lange et al. 2001; Buziol et al. 2002; Visser et al. 2002). The newest development is reported by Schaub et al. (2006) who use a coiled, single tube, heat exchanger connected to the bioreactor to realize a continuous sampling, quenching and extraction procedure.
The crucial point is that all sampling systems need a sampling port submerged into the cultivation broth. Most commonly this sampling port is situated at the bottom of a stainless-steel bioreactor to avoid long residence times between sampling position and cell inactivation. In this work, contrastingly, a new sample system has been developed to overcome this problem, so that intracellular metabolite concentrations can be investigated even in common glass fermenters, having sampling ports only at the lid.
The goal of this work is to investigate the influence of common process parameters on the sampling and quenching procedure including pressure difference in the sampling system, overpressure inside the bioreactor, initial mass load and temperature of the cooling fluid in the sampling system and its composition. At the end, a proposal for the best combination for a reliable fast sample harvest and quenching procedure is given.
Materials and methods
Micro-organism, growth medium and cultivation
Escherichia coli K12 wild type strain (DSM 498, DSMZ, Braunschweig, Germany) multisubstrate continuous cultivations were performed with a feed concentration of 30 g l−1 total carbon with various ratios of glucose, fructose and acetate, as described in detail by Hiller J et al. (Submitted). The cultivations were carried out in a stirred tank bioreactor with a reaction volume of 3 l. Samples for steady state experiments were taken after reaching at least five residence times.
Viability test
Differential permeability of two staining agents (SYTO 9 and propidiumiodid) was used to analyse viability of micro-organisms during the quenching procedure (LIVE/DEAD BacLight, Invitrogen, Karlsruhe, Germany). To analyse viability, a cell suspension, OD600 = 0.05, was incubated for 15 min with both staining agents in a microtiter plate. Fluorescence at 485 nm excitation wavelength and 520 nm and 620 nm reading wavelength was measured. The ratio of both intensities is used as the signal for membrane integrity. Defined mixtures of live and inactivated bacteria were used for calibration.
Sampling device
A newly developed sampling device (Hiller et al. 2003) consisting of a port and a sample container was used to harvest sample and to rapidly stop the metabolism. The sampling device is mounted on the port in the lid of a lab-scale bioreactor so that the sampling position is right amidst the cultivation broth inside the reactor. The tubular sampling probe is closed with a quick coupling at the lower end (Fig. 1). The sample container is closed with a corresponding quick coupling at one end and with a lid on its top (Fig. 2). Before taking a sample, the container is filled with a mixture of methanol/water (60:40, v/v.) as quenching liquid, the pressure was reduced to about 200 mbar and the container was cooled down to −50°C at the maximum in an ethanol bath. For sampling, the container is introduced into the tubular probe and pressed down so that the valve at the bottom of the container is coupled to the valve at the bottom of the probe and both valves are simultaneously opened. Immediately the sample is transported through the guiding tube directly into the inactivation fluid driven by the pressure difference and is inactivated. By withdrawing the container from the tubular probe the internal valves are sealed without external contamination of the fluids.
Optimisation of sampling procedure
To characterize the sampling operation the sample container was pre-filled with varying volumes of three different methanol solutions (methanol/water 60:40, v/v) (a) without buffer, (b) buffered to a pH of 7.5 with 30 mM HEPES and, (c) buffered to a pH of 7.5 with 70 mM HEPES). The pressure inside the container was reduced to (a) 200 mbar, (b) 400 mbar and (c) 600 mbar. The container was cooled afterwards in an ethanol bath to −30, −40°C or −50°C. During sampling the temperature change at the inlet of the sample container was monitored by a thermo-couple as indicated in Fig. 2.
The quenched cell suspension was transferred into a test tube and centrifuged (6,000g, 3 min, −19°C) to separate intra- and extra-cellular metabolites. Supernatant was stored at −20°C until analysis.
Results and discussions
Rapid sampling and quenching device
The influence of process parameters in the dynamic operation of the sampling system was characterized via an experimental design using the D-optimal criterion. The goal of the design was to find the appropriate operational conditions which maximized the total sample load (MS), but which minimized the total temperature change of the sample (ΔTS). MS and ΔTS were taken as design variables since obtaining small harvest do not assure enough mass sample for metabolite concentrations analysis and obtaining high temperature changes might not assure the immediate inactivation of any metabolic activity. The parameters considered to characterize the operation were:
-
1.
Pressure difference in the sampling system (X1 = ΔP)
-
2.
Overpressure inside the bioreactor (X2 = OP)
-
3.
Initial mass load of cooling fluid in the sampling system (X3 = M0)
-
4.
Initial temperature of the cooling fluid (X4 = T0)
-
5.
Composition of the cooling fluid (X5 = HEPES)
The optimization model for the design variables is given by:
and,
where α and β are the corresponding coefficients for the models.
Table 1 lists the five encoded and centred values of the operational parameters (three levels).
The total sample load (MS) is depicted in Fig. 3 as function of the independent variables ΔP, OP, M0, T0 and HEPES after thermal equilibrium was reached asymptotically. This equilibrium was observed experimentally for every assay after maximal 10 s. After thoughtful analysis of the experimental design results, it was asserted that only the main effects of the five independent variables were significant. That is, a reduction of Eqs. 1 and 2 to linear models was suited enough to accurately approximate the design variables without taking into account interaction or second order dependences.
Total sample load as function of the pressure difference in the sampling system (ΔP), the overpressure inside the bioreactor (OP), the initial mass load of cooling fluid in the sampling system (M0), the initial temperature of the cooling fluid (T0) and the composition of the cooling fluid (HEPES). (···) Mean value of the total sample load, (- - -) 95% confidence interval, (↓) optimal operational point
From Fig. 3 can be infer that the total sample load (MS) is most sensitive to changes in the initial mass load of cooling fluid (M0) but almost independent to changes of the overpressure inside the bioreactor (OP).
For the case of the total temperature change of the sample (ΔTS), the initial mass load of cooling fluid in the sampling system (M0), the initial temperature of the cooling fluid (T0) and the composition of the cooling fluid (HEPES) were the most influencing parameters (see Fig. 4).
Total temperature change of the sample on the basement of the sampling device after 10 s of probing (thermal equilibrium). Sample load as function of the pressure difference in the sampling system (ΔP), the overpressure inside the bioreactor (OP), the initial mass load of cooling fluid in the sampling system (M0), the initial temperature of the cooling fluid (T0) and the composition of the cooling fluid (HEPES). (···) Mean value of the total sample load, (- - -) 95% confidence interval, (↓) optimal operational point
Taking into account only the linear dependences described by Eqs. 1 and 2, the model predicts a maximal value of 6.06 ± 2.67 g of total sampling load (MS), and a minimal value of −21.68 ± 3.96°C for the total temperature change of the sample (ΔTS), i.e. for a final equilibrium temperature inside the sampling system of −18.32 ± 3.96°C (TFinal = T0 − ΔTS). These values are obtained establishing the preliminary specifications for operation of the sampling device:
-
Pressure difference in the sampling system (ΔP) = 800 mbar (maximum), obtaining maximal MS and almost no sensitivity for ΔTS.
-
Overpressure inside the bioreactor (OP) = 200 mbar (minimum), with almost no sensitivity for MS, and with almost no sensitivity for ΔTS (compared to other parameters).
-
Initial mass load of cooling fluid in the sampling system (M0) = 25 g (centre), since the effects for MS and for ΔTS are antagonistic.
-
Initial temperature of the cooling fluid (T0) = −40°C (centre), since even when the effects for MS and for ΔTS are not antagonistic, extreme values cause an increment in the variances of the design variables.
-
Composition of the cooling fluid (HEPES) = 35 mM (centre), since the effects for MS and for ΔTS are antagonistic.
Direct dynamical temperature measurements inside the device were performed as well. This yielded, in some cases, a dynamical lumped oscillating behaviour. The phenomenon was observed in most of the design experiments where the cooling fluid lacked of HEPES (Fig. 5). As reported by Buchholz et al. (2001), the presence of HEPES in the cooling fluid allows the use of the quenching fluid at −50°C (avoiding the formation of ice crystals). It is assumed that solid ice crystals and cooling fluid abruptly melt and mix as soon as the bioreactor sample flows into the device. The combined melting and mixing processes might be responsible for the lumped oscillating temperatures: while the inflowing sample causes an increase in the temperature of the mixture, the melting of solid ice tends to reduce it absorbing heat from the mixture until thermal equilibrium is reached asymptotically.
Since it was desirable to assure optimal mixing and heat transfer between sample and cooling fluid, lumped oscillating operation was avoided using HEPES—keeping the presence of the icy phase minimal.
The characterization of the sample inflow rate (r M) was done from energy and mass balances around the device. Modelling of the sampling process was carried out considering mixture with no phase change and no heat interchange from the device to its surroundings (adiabatic operation: Wagner 1981). An ideal thermal capacity for the resulting mixture as linear function of its mass composition (Perry and Chilton 1973), methanol/sample was taken into account assuming the sample properties as those of water.
Since the true sample inflow rate is not known a priori, several feasible patterns which fulfil the mass and thermal balances must be tested. A systematic way to generate these patterns can be attained using an artificial neural network (ANN). Through the neural network a non-linear relationship between the sample inflow rate and operational time is established such that, r M = f ANN(t). The neural network can mimic the feasible pattern of the true sample inflow rate after a training procedure, which consists on the iterative identification of the neural network parameters with a genetic algorithm. The minimization of the least squared sum of residuals between measured and modelled temperatures (Fig. 6) was used as identification criteria. A detailed description of the neural network architecture, as well as the genetic algorithm specifications can be found in Franco-Lara and Weuster-Botz (2005).
Characterization of the sample inflow rates. Smooth continuous non-linear rate patterns are generated with a neural network. After estimating the corresponding mass and thermal balances, the simulated and measured temperature patterns are compared and used to minimize the least squared sum of their residuals (LSSR) by means of a genetic algorithm (Franco-Lara and Weuster-Botz 2005). G corresponds to the maximal number of iteration loops performed (50)
Figure 7 depicts the result obtained for the mixture’s temperature change and also the estimation of the corresponding mass inflow rate and total mass inside the sampling device. For most of the experiments, the maximal mass inflow rate was about 3.4 g s−1, presenting first a constant maximal phase, followed by a rapid descent and a final near-zero state. An asymptotic trend for the temperature equilibrium can be stated after approximately 4 s of sampling start.
Influence of quenching on viability and metabolism
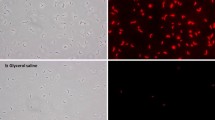
Since there is evidence that the quenching step could lead to cell leakage of bacteria as reported by Wittmann et al. (2004) the influence of the quenching step on the viability of the micro-organisms was analysed. The test revealed that 99.1 ± 0.8% of the cells are viable and are not permeabilised after quenching. Cell size and cell volume did not change significantly as well.
To investigate the effect of prolonged storage of the cells in quenching solution the change in metabolite concentrations during storage in the quenching solution at −15°C was measured up to 45 min. It was found that the concentrations of all investigated metabolites did not vary significantly for all extraction procedures during storage (data not shown). Furthermore, the supernatant was concentrated five fold by freeze drying of 1 ml and resuspending the lyophilisate in 200 μl of quenching solution. In this concentrated solution none of the investigated metabolites was detected. Since cells are stored just a few minutes in the quenching solution until the centrifuged cell pellet and the supernatant are separated, no leakage of metabolites is assured.
Concluding remarks
A novel rapid in-situ sampling and quenching device applicable to laboratory glass fermenters accessible from the top was developed and its operation characterized and optimized. The system was conceived to analyse steady state metabolite concentrations through manual sample operation. With a dead volume of about 700 μl and a flow rate it, enabling thus a fast inactivation of metabolism and a reliable quantification of intracellular concentrations of intermediates in microorganisms. Neither metabolite leakage nor negative influences from the quenching procedure or quenching solution on cell viability were detected.
References
Buchholz A, Takors R, Wandrey C (2001) Quantification of intracellular metabolites in Escherichia coli K12 using liquid chromatographic-electrospray ionization tandem mass spectrometric techniques. Anal Biochem 295:129–137
Buziol S, Bashir I, Baumeister A, Claaßen W, Noisommit-Rizzi N, Mailinger W, Reuss M (2002) New bioreactor-coupled rapid stopped-flow sampling technique for measurements of metabolite dynamics on a subsecond time scale. Biotechnol Bioeng 80(6):632–636
Chassagnole C, Noisommit-Rizzi N, Schmid JW, Mauch K, Reuss M (2002) Dynamic modeling of the central carbon metabolism of Escherichia coli. Biotechnol Bioeng 79(1):53–73
De Koning W, van Dam K (1992) A method for the determination of changes of glycolytic metabolites in yeast on a subsecond time scale using extraction at neutral pH. Anal Biochem 204:118–123
Franco-Lara E, Weuster-Botz D (2005) Estimation of optimal feeding strategies for fed-batch bioprocesses. Biopro Biosys Eng 27:255–262
Hajjaj H, Blanc BJ, Goma G, Francois J (1998) Sampling techniques and comparative extraction procedures for quantitative determinations of intra- and extracellular metabolites in filamentous fungi. FEMS Microbiol Lett 164:195–200
Hiller J, Schwaiger B, Weuster-Botz D (2003) Probenahmesystem für fluide Proben. German patent application DE 103 14 512.5, 21 Oct 2004
Lange HC, Eman M, van Zuijlen G, Visser D, van Dam JC, Frank J, Teixeira de Mattos MJ, Heijnen JJ (2001) Improved rapid sampling for in vivo kinetics of intracellular metabolites in Saccharomyces cerevisiae. Biotech Bioeng 75(4):406–415
Larsson G, Törnkvist M (1996) Rapid sampling, cell inactivation and evaluation of low extracellular glucose concentrations during fed-batch cultivation. J Biotech 49:69–82
Perry RH, Chilton CH (eds) (1973) Chemical engineers’ handbook, 5th edn. McGraw-Hill Book Inc., New York
Schäfer U, Takors R, Weuster-Botz D (1999) Automated sampling device for monitoring intracellular metabolite dynamics. Anal Biochem 270:88–96
Schaub J, Schiesling C, Reuss M, Dauner M (2006) Integrated sampling procedure for metabolome analysis. Biotechnol Prog 22:1434–1442
Theobald U, Mailinger W, Reuss M, Rizzi M (1993) In vivo analysis of glucose-induced fast changes in yeast adenine nucleotide pool applying a rapid sampling technique. Anal Biochem 214:31–37
Visser D, van Zuylen GA, van Dam JC, Oudshoorn A, Eman MR, Ras C, van Gulik WM, Frank J, van Dedem GWK, Heijnen JJ (2002) Rapid sampling for analysis of in vivo kinetics using the bioscope: a system for continuous-pulse experiments. Biotechnol Bioeng 79(6):674–680
Wagner W (1981) Wärmeübertragung: Grundlagen. Vogel-Verlag, Würzburg
Weuster-Botz D (1997) Sampling tube device for monitoring intracellular metabolite dynamics. Anal Biochem 246:225–233
Wittmann C, Krömer JO, Kiefer P, Binz T, Heinzle E (2004) Impact of the cold shock phenomenon on quantification of intracellular metabolites in bacteria. Anal Biochem 327:135–139
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hiller, J., Franco-Lara, E., Papaioannou, V. et al. Fast sampling and quenching procedures for microbial metabolic profiling. Biotechnol Lett 29, 1161–1167 (2007). https://doi.org/10.1007/s10529-007-9383-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-007-9383-9









 )
) 
