Abstract
A novel expression vector (pLR) driven by hup promoter and Bifidobacterium β-galactosidase signal peptide was constructed. The pLR vector was used for the expression of the optimized human IL-10 synthetic gene in Escherichia coli and Bifidobacterium longum. In both microorganisms, rhIL-10 was in a soluble form in total extract cells. The recombinant hIL-10 was partially processed in E. coli, whereas in Bifidobacterium all rhIL-10 was found in the mature form.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Delivery systems based on non-pathogenic bacteria have several advantages over DNA vaccines: transduction of the therapeutic gene into the human cell genome is not necessary and target gene expression can be stopped at any time by administration of suitable antibiotics. Moreover, a bacterial delivery system is not dependent on the presence of the tumor-specific antigens and could be applied to all patients that present tumor hypoxic/necrosis regions.
Bifidobacterium spp. are Gram-positive anaerobic bacteria and an predominant members of intestinal microflora that play important role in health promoting properties (Guarner and Malagelada 2003). Their economic importance is beyond doubt; these microorganisms are widely used in the fields of food science, medicine and industry. Using non-pathogenic microorganisms as delivery systems for cancer gene therapy (Yazawa et al. 2001) have increased since have been demonstrated that Bifidobacterium can selectively colonize and grow on the hypoxic region of solid tumors after intravenous injection (Kimura et al. 1998). Bifidobacterium has also been evaluated within the context of enzyme prodrug therapy (Fujimori et al. 2002; Theys et al. 2003; Fu et al. 2005; Yi et al. 2005), these data indicates the potential of Bifidobacterium as a tumor-specific gene delivery system for cancer treatment; however, no commercial expression vectors are available for the transformation of these microorganisms.
In this work, we describe the design of a novel binary expression system, named pLR, for E. coli and Bifidobacterium. pLR is driven by the Bifidobacterium hup promoter and terminator sequences. The functionality of the system was tested in both microorganisms by the expression of the human interleukin-10 (hIL-10) synthetic gene. The hIL-10 has been proposed as therapeutic treatment for autoimmune mediated illness such as inflammatory bowel disease (IBD) (deWaal and Moore 1998).
Materials and methods
Bacterial strains and media
E. coli TOP 10 was used for general cloning purposes and was grown at 37°C in Luria-Bertani broth containing at 100 μg ampicillin ml−1. Bifidobacterium longum ATCC 15707 was routinely grown in MRS broth (Difco) supplemented with 0.05% (w/v) l-cysteine at 37°C under anaerobic conditions. Electrocompetent Bifidobacterium cells were obtained following the methodology of Argnani et al. (1996). Transformant cells were selected in MRS media containing 10 μg chloramphenicol ml−1. The production of recombinant human interleukin-10 (rhIL-10) was made in MRS medium buffered at pH 8 using filter sterilized 50 mM carbonate solution (Schotte et al. 2000).
Construction of expression vector
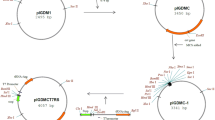
The promoter and terminator sequences from hup gene were obtained from the Bifidobacterium longum NCC2705 genome database (GenBank AEO14295). The β-galactosidase signal peptide sequence (BIF3) from B. bifidum (GeneBank Access No. AJ224435) was fused in frame to hIL-10 gene. The construction denominated as “expression cassette” consisted of hup promoter, BIF3 signal peptide, hIL-10 optimized gene and hup terminator sequences, was synthesized and ligated into a shuttle vector pCR4 to yield pCR4-796 (Entelechon). The expression cassette was excised from pCR4-796 by EcoRI restriction and ligated into pDG7 EcoRI restricted plasmid (Fig. 1). The resulting plasmid of 8.0 kb was named as pLR (Mexican Patent Pending).
Expression of human Interleukine-10
E. coli TOP10 strain transformed with pLR was inoculated in LB medium containing 100 μg ampicillin ml−1 and incubated overnight at 37°C. Cells were separated by centrifugation, the pellet was resuspended and sonicated in TNT buffer (50 mM Tris/HCl pH 8, 300 mM NaCl, 0.1% Triton X-100) and centrifuged at 1500g for 10 min. Proteins from the supernatant were precipitated with five volumes of methanol and subsequently dissolved in Laemmli sample buffer (Schotte et al. 2000).
Transformed B. longum was grown for 48 h in MRS broth with chloramphenicol, and fresh broth was inoculated with this culture. After 3 h of incubation at 37°C, the cells were centrifuged at 1500g for 10 min and pellets were resuspended in MRS medium buffered with carbonate solution, and incubated for another 6 h. Cells and culture supernatants were separated by centrifugation. Bifidobacterium cell pellets were resuspended in TNT buffer and disrupted with glass beads. Cell debris was separated by centrifugation and supernatants were treated as described above.
Western blot and ELISA analysis
Protein fractions were separated by SDS-PAGE and electroblotted onto nitrocellulose membrane (GE Healthcare) for Western blot analysis. Recombinant hIL-10 (rhIL-10) was detected by immunoblotting and commercial rhIL-10 (Prepro Tech) was used as standard. The polyclonal rabbit anti-human IL-10 (Prepro Tech) was used as the primary antibody diluted to 1:1000. The goat anti-rabbit IgG (H+L) coupled to alkaline phosphatase (Bio-Rad) diluted to 1:2000 was used as a secondary antibody. Enzymatic activity was revealed with NBT/BCIP substrate (Invitrogen). Quantification of rhIL-10 was assayed by ELISA kit (eBiosciences).
Results and discussion
Expression vector construction
Protein expression using the plasmid pLR is driven by the constitutive hup promoter (Takeuchi et al. 2002). The pLR vector contains the B. bifidum β-galactosidase BIF3 signal peptide (Moller et al. 2001) was fused in frame to the codon optimized hIL-10 synthetic gene. The synthetic hIL-10 gene was designed guided by the preferred codons to be expressed in E. coli and Bifidobacterium longum. The resultant optimized gene had 81% identity with respect to the wild-type gene (Fig. 1). A summary of codon preference in E. coli and Bifidobacterium longum and codon usage in the wild type and the optimized synthetic hIL-10 gene is shown in Table 1, where the most significant changes were for arginine, leucine, proline and serine codons. The restriction sites, SalI and NcoI (upstream signal peptide), Eco47III between the signal peptide and hIL-10 gene, and SmaI and SphI (downstream the gene), were included to facilitate the subcloning of other target genes in pLR (Fig. 2). Clones where the expression cassette was ligated in the same direction as the bla gene and clones in contrary sense were named pLR1 and pLR2, respectively (Fig. 2). E. coli was transformed with each pLR constructions. After HindIII–SmaI double restriction, one fragment of 720 bp was obtained in pLR1 clones (Fig. 3, lane 2), and as expected no fragment were observed with pLR2 clones (Fig. 3, lane 3).
Esquematic representation of pLR plasmid. The HU promoter and terminator, the signal peptide BIF3 was fused to the synthetic hIL-10 gene. When the expression cassette was ligated in the sense of bla gene was named as pLR1, contrary to gene bla was named as pLR2 (zoomed image). pMB1 represents the replicon for Bifidobacterium; Ori, E. coli origin; bla, Ampicillin resistance; cat, Chloramphenicol resistance
Expression of rhIL-10
Functionality of pLR1 and pLR2 was tested in E. coli. The amount of rhIL-10 attained with pLR1 was 4.2 times more than pLR2, interestingly the recombinant protein was detected in higher amounts in the culture medium than inside of the cells (Table 2). Western blot analysis from cell pellets showed two forms of rhIL-10 in E. coli, the 18.6 kDa band represents the mature form, while the higher molecular weight band corresponds to the non-processed protein (Fig. 4A).
Immunoblot detection for rhIL-10 in (A) E. coli and (B) Bifidobacterium longum. (A) Lane 1, rhIL-10 standard; Lanes 2–7, rhIL-10 expressed from different E. coli clones transformed with pLR1; lane 8, control expression, E. coli clone with pLR without hIL-10 gene. (B) Lane 1, rhIl-10 standard; lane 2 and 3 rhIL-10; lane 4, Bifidobacterium clone with pLR without hIL-10 gene; lane 5, MW markers
In the case of B. longum, only transformants with pLR2 were obtained. It has been claimed that the presence of a thick (multi-layered) cell wall generally forms a barrier for the uptake of exogenous DNA molecules; this gives the low efficiency of Bifidobacterium transformation (Argnani et al. 1996). As shown on Fig. 4B, all protein expressed for B. longum was correctly processed; only the 18.6 kDa protein was observed.
The pLR plasmid was useful to express rhIL-10 in both Escherichia coli and Bifidobacterium longum. This is the first report showing that promoters and signal peptides from Bifidobacterium are recognized for E. coli. Comparative analysis from E. coli and Bifidobacterium promoters showed that -35, -10 box and ribosome binding sites (RBS) shares a high degree of homology (Takeuchi et al. 2002), this homology could explains the functionality of Bifidobacterium hup promoter in E. coli. Since the concentration of mature rhIL-10 in B. longum was low (22 pg ml−1), the optimization of expression conditions is further recommended for larger scale production. However, high amounts of IL-10 could have secondary effects; the IL-10 produced for B. longum could be enough for an in situ delivery. Recombinant proteins in B. longum can bind to the outside of the cell wall (Rhim et al. 2006) and this could be the reason why IL-10 was not detected in the culture medium. This will be important in the design of more functional system for in situ pro-drug directed therapy. Further studies for immunolocalization should be carried out. Several others proteins such as β-galactosidase, lipase, amylase, among others therapeutic proteins, could be achieved with pLR plasmid for in vivo delivery.
The alternative approach with more potential to specifically target cancer cells are based on the use of non-toxic bacteria as Bifidobacterium. Further improvements of pLR systems could be achieved by incorporating stronger inducible promoters.
References
Argnani A, Leer RJ, van Luijk N, Pouwels PH (1996) A convenient and reproducible method to genetically transform bacteria of the genus Bifidobacterium. Microbiology 142:109–114
de Waal MR, Moore KW (1998) Interleukin-10. In: Thomson A (ed) The cytokine handbook, 3rd edn. San Diego, CA, Academic Press, pp 333–364
Fu GF, Li X, Hou YY, Fan YR, Liu WH, Xu GX (2005) Bifidobacterium longum as an oral delivery system of endostatine for gene therapy on solid liver cancer. Cancer Gene Ther 12:133–140
Fujimori M, Amano J, Taniguchi S (2002) The genus Bifidobacterium for cancer gene therapy. Curr Opin Drug Discov Devel 5:200–203
Guarner F, Malagelada JR (2003) Gut flora in health and disease. Lancet 361:512–519
Kimura NT, Taniguchi S, Aoki K, Baba T (1998) Selective localization and growth of Bifidobacterium bifidum in mouse tumors following intravenous administration. Cancer Res 40:2061–2068
Moller PL, Jorgensen F, Hansen OC, Madsen SM, Stougaard P (2001) Intra and extracellular β-galactosidases from Bifidobacterium bifidum and B. infantis: Molecular cloning, heterologous expression, and comparative characterization. Appl Environ Microbiol 67:2276–2283
Rhim SL, Park MS, Ji GE (2006) Expression and secretion of Bifidobacterium adolescentis amylase by Bifidobacterium longum. Biotechnol Lett 28:163–168
Schotte L, Steidler L, Vandekerckhove J, Remaut E (2000) Secretion of biologically active murine interleukin-10 by Lactococcus lactis. Enzyme Microbiol Technol 27:761–765
Takeuchi A, Matsumura H, Kano Y (2002) Cloning and expression in Escherichia coli of a gene, hup, encoding the histone-like protein HU of Bifidobacterium longum. Biosci Biotech Biochem 66:598–603
Theys J, Barbé S, Landuyt S, Nuyts L, Mellaert V, Wouters B, Anné J, Lambin P (2003) Tumor specific gene delivery using genetically engineered bacteria. Curr Gene Ther 3:207–221
Yazawa K, Fujimori M, Nakamura T, Sasaki T, Amano J, Kano Y, Taniguchi S (2001) Bifidobacterium longum as a delivery system for gene therapy of chemically induced rat mammary tumors. Breast Cancer Res Treat 66:165–170
Yi C, Huang Y, Guo ZY, Wang S (2005) Antitumor effect of cytosine deaminase/5-flurocytosine suicide gene therapy system mediated by Bifidobacterium infantis on melanoma. Acta Pharmacol Sin 26:629–634
Acknowledgments
María de Lourdes thanks to CONACyT for the scholarship no. 157707. Special thanks to Dr. Matteuzzi for pDG7 vector. To Leticia Santos (Helsinki University) and Michael Raisor (Texas A&M) for the critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reyes Escogido, M.L., De León Rodríguez, A. & Barba de la Rosa, A.P. A novel binary expression vector for production of human IL-10 in Escherichia coli and Bifidobacterium longum . Biotechnol Lett 29, 1249–1253 (2007). https://doi.org/10.1007/s10529-007-9376-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-007-9376-8