Abstract
Supplementation with CaCl2·2H2O (50 mg l−1) or CuSO4·5H2O (10 mg l−1) improved mannitol production by Candida magnoliae by 14.5 and 18.6% (25 and 32 g/L), respectively. When used in combination, they acted synergistically: Ca2+ decreased the intracellular concentration of mannitol 30%, whereas Cu2+ increased the intracellular activity of mannitol dehydrogenase 1.6-times more than control. Ca2+ probably works by altering the permeability of cells to mannitol, whereas, Cu2+ increases the activity of an enzyme responsible for mannitol biosynthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mannitol is found in bacteria, algae, fungi, and higher plants (Lewis and Smith 1967). Acyclic polyols, including mannitol, are important for growth, carbon storage, recycling of reductants, efficient carbon fixation, and the stress tolerance of microorganisms and higher plants (Pharr et al. 1995, Smirnoff and Cumbes 1989). Mannitol is about half as sweet as sucrose and is not metabolized by humans; it is, therefore considered a low-calorie sweetener (Furia 1972).
Mannitol has been produced by chemical hydrogenation of fructose. This method yields almost equal amounts of mannitol and a byproduct, its isomer sorbitol (Makkee et al. 1985). The resulting need to separate mannitol from its isomer, coupled with the very low conversion yield of the process, results in a high cost of production. To overcome these problems, many approaches to produce mannitol via microbiological techniques are being studied (Wisselink et al. 1990, Wong et al. 1990, Yun and Kim 1998). However, microbiological production of mannitol on an industrial scale has been prevented by low yield and productivity. Recently, we isolated a high-mannitol-producing yeast strain from fermentation sludge and identified the new isolate as Candida magnoliae HH-01. In a previous paper (Song et al. 2002), we reported the characterization of C. magnoliae HH-01 strain and the highest mannitol production among mannitol-producing microorganisms. We also characterized a novel mannitol dehydrogenase (MDH) from C. magnoliae HH-01 (Lee et al. 2003).
Minerals have been reported to influence the production of some sugar alcohols and the activity of enzymes involved in sugar alcohol synthesis (Lee et al. 2000, 2002, Kim and Oh 2003), they are economical and easy to deliver during liquid fermentation. However, their effect on mannitol production has not been studied. In the current study, we screened minerals for a stimulatory effect on the production of mannitol by C. magnoliae and then investigated the effect of selected minerals on the activity of relevant enzymes in supplemental experiments.
Materials and methods
Microorganism and media
Candida magnoliae HH-01 (KCCM-10252) (Song et al. 2002) was grown in the medium containing 20 g glucose l−1 and 10 g yeast extract l−1. The fermentation medium consisted of 20 g glucose l−1, 10 g yeast extract l−1, 1.2 g urea l−1, 2.5 g KH2PO4 l−1, 0.4 g MgSO4·7H2O l−1, and minerals (see Table 1) such as Ca2+ and Cu2+..
Culture conditions
A suspension of frozen cells was inoculated into a 500 ml flask containing 75 ml growth medium and incubated at 30°C and 250 rpm for 24 h. The culture broth of 5% (v/v) was transferred, either to a 500 ml flask containing 75 ml fermentation medium or to a 5 l fermenter (KoBiotech Co., Incheon, Korea) containing 3 l fermentation medium. Flask experiments were performed at 35°C and 220 rpm in a shaker; the pH of the flask culture was not controlled. After incubation for 24 h, glucose and fructose were fed to give 10 g l−1 and 150 g l−1, respectively. When fructose was added, the pH was around 5.5. After further incubation at 35°C for 120 h, the amount of mannitol produced was measured by HPLC. Fermenter experiments were performed at 35°C; the pH of the culture broth was automatically controlled at 5.0 through the addition of 1 m HCl or NaOH by a peristaltic pump.
When the cell mass reached 12 g l−1, the agitation speed and aeration rate were adjusted to 500 rpm and 0.5 vvm, respectively, and then reduced to 250 rpm and 0.0 vvm, respectively, to limit the dissolved O2 concentration (DO). DO is a key variable in this process for conversion of fructose to mannitol (Song et al. 2002). Simultaneously with the limitation of DO, the carbon source, the mixed solution of fructose (250 g l−1) and glucose (30 g l−1), was fed.
Enzyme assay of mannitol dehydrogenase (MDH)
Washed cells from the culture broth were resuspended in disruption buffer containing 20 mM Tris/HCl (pH 7.8), 10 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, and 1 mM PMSF, and ground with glass beads (0.5 mm diam). The MDH assay (Lee et al. 2003) mixture (1 ml) consisted of 0.25 mM NADPH, 0.1 M fructose, and cell extract (1.2 g protein/ml and 10%) in 20 mM Tris/HCl (pH 7.8). One unit of enzyme activity represents 1 μmol NADPH consumed per min (monitored at 340 nm).
Analytical methods
The concentration of DO in the liquid phase was monitored with an Ingold polarographic electrode. Optical density (OD) was measured at 660 nm, and dry cell weight was determined after the culture broth was centrifuged at 6,000 × g, washed with distilled water, and dried overnight at 105°C. One OD unit was found to be equivalent to 0.41 ± 0.03 g (dry cell wt) l−1. The concentrations of mannitol, fructose, and glucose were determined by HPLC coupled to a refractive index detector. The column (Carbohydrate Column, 4.6 mm × 250 mm, WATO44355, Waters, MA, USA) was eluted with acetonitrile/H2O (85:15, v/v) at 30°C and at 1.5 ml/min. Protein was determined by the Lowry method using bovine serum albumin as the standard.
Results and discussion
Effect of minerals on mannitol production
The effect of various metal ions on mannitol production, by C. magnoliae is shown in Table 1. Ca2+ and Cu2+ increased production and also had a synergistic effect when added together (Table 2). Mannitol was maximal at 96.5 g l−1 when both Ca2+ and Cu2+ were added together.
Increased mannitol production with supplemental Ca2+ and Cu2+
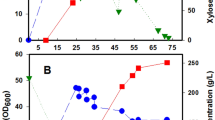
To investigate in more detail the effects of Ca2+ and Cu2+ on mannitol production, the metal ions were added to a culture growing in a stirred tank reactor. There were no marked differences in cellular growth between the control and the supplemented cultures, but the supplemental Ca2+ and Cu2+ significantly enhanced mannitol production as shown in Figure 1. After culture for 130 h, the final mannitol concentration in the control was 172 g l−1. This increased to 197 g l−1 with supplemental Ca2+, 204 g l−1 with supplemental Cu2+ and 223 g l−1 with the combination of both Ca2+ and Cu2+. The volumetric rate of mannitol production of control culture was 1.32 g l−1 h−1, compared with 1.52 g l−1 h−1 with Ca2+ supplementation, 1.57 g l−1 h−1 with Cu2+ supplementation, and 1.72 g l−1 h−1 with combined Ca2+ and Cu2+ supplementation.
Cell growth and sugar consumption during mannitol formation by C. magnoliae. Cell growth in culture without mineral (◆), culture with Ca2+ (■), culture with Cu2+ (▼), and culture with Ca2+ and Cu2+ (●); sugar consumption in culture without minerals (◊), culture with Ca2+ (□), culture with Cu2+ (▽), and culture with Ca2+ and Cu2+ (○). B. Mannitol production by C. magnoliae. Mannitol production in culture without mineral (◆), culture with Ca2+ (■), culture with Cu2+ (▼), and culture with Ca2+ and Cu2+ (●). CaCl2·2H2O (50 mg l−1) and CuSO4·5H2O (10 mg l−1) were added separately or together
To investigate the roles of Ca2+ and Cu2+ in mannitol production, the intracellular mannitol concentration and the activity of MDH were measured. The intracellular mannitol concentration in the culture with supplemental Ca2+ decreased 30%, but remained unchanged with supplemental Cu2+. MDH activity was 1.6 times more with supplemental Cu2+ than in control, but was not affected by supplemental Ca2+ (Table 2). Divalent ions such as Ca2+ and Cu2+ have been reported to influence the permeability of membrane (Zhao et al. 1987) and the production of some polyols (Kim and Oh 2003, Lee et al. 2000). Similar stimulatory effect of Cu2+ ion on the activity of erythrose reductase, a key enzyme for erythritol production, was reported (Lee et al. 2002).
Conclusion
These results suggest that whereas Ca2+ alters the permeability of C. magnoliae to mannitol, Cu2+ does not. Cu2+ increases the activity of MDH in cells, whereas Ca2+ has no effect on enzyme activity. Thus Ca2+ increased the egress of intracellular mannitol, while Cu2+ increased the activity of intracellular MDH. The highest production of mannitol resulted from the synergistic effect of Ca2+ on cell permeability and that of Cu2+ on biosynthetic enzyme activity. The mannitol yield with C. magnoliae HH-01, from a total of 250 g fructose/L, was 89% when Ca2+ and Cu2+ were added together. No other paper has reported such a high mannitol yield. Our results improve the understanding of mannitol biosynthesis in C. magnoliae and should contribute to better industrial production of mannitol by biological processes.
References
Furia TE (1972). In: Bergmeyer H. U. (ed) Handbook of Food Additives, 2nd edn, Cleveland:CRC Press, pp. 431–455
Kim TB, Oh DK (2003) Xylitol production by Candida tropicalis in a chemically defined medium. Biotechnol Lett 25:2085–2088
Lee JK, Ha SJ, Kim SY, Oh DK (2000) Increased erythritol production in Torula sp. By Mn2+ and Cu2+. Biotechnol Lett 22:983–986
Lee JK, Koo BS, Kim SY (2002) Fumarate-mediated inhibition of erythrose reductase, a key enzyme for erythritol production by Torula corallina. Appl Environ Microbiol 68:4534–4538
Lee JK, Koo BS, Kim SY, Hyun HH (2003) Purification and characterization of a novel mannitol dehydrogenase from a newly isolated strain of Candida magnoliae. Appl Environ Microbiol 69:4438–4447
Lewis DH, Smith D C (1967) Sugar alcohols (polyols) in fungi and green plants. I. Distribution, physiology and metabolism. New Phytol 66: 143–184
Makkee M, Kieboom APG, Bekkum HV (1985) Production methods of D-mannitol. Starch 37:136–141
Pharr DM, Stoop JMH, Studer-Feusi ME, Wiliamson JD, Massel MO, Cokling MA. z1995) In: Madore, M. A. and Lucas, W. J. (eds) Current Topics in Plant Physiol American Society of Plant Physiologists, Vol. 13 Rockville, MD, , p. 180
Smirnoff N, Cumbes QJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28:1057
Song KH, Lee JK, Song JY, Hong SG, Baek H, Kim SY, Hyun HH (2002) Production of Mannitol by a novel strain of Candida magnoliae HH-01. Biotech Lett 24:9–12
Wisselink HW, Weusthuis RA, Eggink G., Hugenholtz J, Grobben GJ (1990) annitol production by lactic acid bacteria: a review. International Dairy Journal 12:151–161
Wong B, Perfect JR, Beggs S, Wright KA (1990) roduction of the hexitol D-mannitol by the pathogenic fungus Cryptococcus neoformans in vitro and in rabbits with experimental meningitis. Infect Immun 58:1664–1670
Yun JW, Kim DH (1998) comparative study of mannitol production by two lactic acid bacteria. J Ferment Bioeng 85:203–208
Zhao XJ, Sucoff E, Stadelmann EJ (1987) l3+ and Ca2+ Alteration of Membrane Permeability of Quercus rubra Root Cortex Cells. Plant Physiol 83:159–162
Acknowledgment
This paper was supported by Konkuk University in 2006.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, JK., Oh, DK., Song, HY. et al. Ca2+ and Cu2+ supplementation increases mannitol production by Candida magnoliae . Biotechnol Lett 29, 291–294 (2007). https://doi.org/10.1007/s10529-006-9230-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-006-9230-4