Abstract
Long-chain acyl-CoA synthetase (LACS) is one of the key enzymes involved in fatty acid metabolism, including phospholipid biosynthesis, triacylglycerol (TAG) biosynthesis, and fatty acid β-oxidation in plants. However, the characterization of LACSs family in seed oil biosynthesis of Brassica napus (B. napus) remains unknown. In the present study, we performed a comprehensive genome-wide analysis of this gene family in B. napus, and 34 B. napus LACS genes (BnaLACSs) were identified. Phylogenetic analysis classified the BnaLACS proteins into four groups (A, B, C, and D), which were supported by highly conserved gene structures and consensus motifs. RNA-Sequencing (RNA-Seq) and qRT-PCR combined analysis revealed that 18 BnaLACSs (BnaLACS1-2, 1–3, 1–4, 1–9, 1–10, 2–1, 2–2, 4–1, 4–2, 6–1, 6–2, 6–4, 7–1, 7–2, 8–1, 8–2, 9–3, and 9–4) were highly expressed in developmental seeds. Comparative expression analysis between extremely high oil content (P1-HO) and low oil content (P2-LO) B. napus cultivars revealed that BnaLACS6-4, BnaLACS9-3, and BnaLACS9-4 may be involved in fatty acid synthesis in chloroplast, and BnaLACS1-10 and 4–1 may play a vital role in lipid biosynthesis in B. napus, which is important for further seed oil accumulation in oilseed rape. The present study provides important information for functional characterization of BnaLACSs in seed oil metabolism in B. napus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fatty acids (FAs) are not only essential for membrane constituents, but also are necessary for plant growth, development, and performance. In the process of fatty acid biosynthesis, long-chain acyl groups (C16-ACP and C18-ACP) were synthesized by sequential condensation of two-carbon units in chloroplasts, and then they can be hydrolyzed by fatty acyl thioesterase A/B (FATA/B) to release free fatty acids which are eventually activated to form acyl-CoA esters by a long-chain acyl-CoA synthetase (LACS) and then exported to the endoplasmic reticulum for generating triacylglycerols (TAGs) (Li-Beisson et al. 2010). Therefore, LACSs play pivotal roles in fatty acid (FA) metabolism (Groot et al. 1976; Tan et al. 2014).
In previous studies, the role of LACSs during lipid biosynthesis was well studied in bacteria (Overath et al. 1969; Black et al. 1992), yeast (Saccharomyces cerevisiae) (Black and DiRusso 2007), green algae (Chlamydomonas reinhardtii) (Jia et al. 2016), mammalian cells (Jetter and Kunst 2008), and some plants (e.g., Arabidopsisthaliana) (Shockey et al. 2002; Pulsifer et al. 2012). In A. thaliana, nine LACS genes make up the LACS family (AtLACSs), which participated in the biosynthetic pathways of many fatty acid-derived molecules and were localized in different cellular organelles, such as AtLACS9 was located at the plastid for TAG formation (Shockey et al. 2002; Weng et al. 2010; Zhao et al. 2010); AtLACS1-AtLACS3 involved in aid transmembrane movement of fatty acids and cuticle biosynthesis (Schnurr et al. 2004; Lu et al. 2009; Pulsifer et al. 2012). It was suggested that members of LACS gene family were functionally redundant in lipid metabolism. For example, AtLACS1 partially overlapped with that of AtLACS9 in seed oil biosynthesis (Schnurr et al. 2002; Zhao et al. 2010); AtLACS4 and AtLACS9 have a strong overlapping function in lipids trafficking between ER and plastid (Jessen et al. 2015). Moreover, the AtLACSs tend to have a non-conservative and tissue-specific expression pattern, such as AtLACS1, AtLACS2, AtLACS4, and AtLACS9, and showed higher expression levels in developing seeds, whereas AtLACS5 was only expressed in flowers (Shockey et al. 2002). Similarly, homologs of LACS were also well proved to have key roles in fatty acid (FA) metabolism. In cotton, GhACS1 and GhACS2 were proved to be anther-specific and GhACS1 was a prerequisite for normal microsporogenesis in early anther development (Wang and Li 2009). In soybean, GmLACS was required for the synthesis of major fatty acids, such as linoleic acid (C-18:3) and oleic acid (C-18:1) (Jang et al. 2015). In Ricinus communis, RcLACS2 could preferentially activate ricinoleate to its CoA thioester to be used for synthesis of TAG (He et al. 2007).
Brassica napus (B. napus) is a worldwide oil crop that can be used for direct human consumption and as a source of biofuel, and thereby is regarded as a promising renewable energy crop (Jian et al. 2016). As compared to Canada and Europe (45–50%), the oil content of B. napus is about 40% in the main producing area of the Yangtze River Basin of China. Therefore, there is still great potential to improve the oil content of B. napus varieties in China. In B. napus, two LACSs homologous genes (BnLACS4 and ACS6) were isolated and proved to be involved in lipid biosynthesis and oil accumulation (Pongdontri and Hills 2001; Tan et al. 2014). Despite this, the functions of the whole LACS gene family in B. napus remain unclear. We are still confused about the roles of LACSs in B.napus, especially their roles in plastidial fatty acid export for TAG formation. Therefore, a systematic research on the entire LACS gene family in B.napus is necessary for screening key Brassica napusLACS genes (BnaLACSs) that play a key role in plastidial fatty acid transport for TAG synthesis. The allotetraploid B. napus was formed by the spontaneous hybridization between B. rapa and B. oleracea approximately 7500 years ago (Chalhoub et al. 2014). Therefore, in the current study, we analyzed the evolutionary relationship of the BnaLACS gene family in B. napus, B. rapa, and B. oleracea. Meanwhile, to focus on the BnaLACS gene family, two cultivars with extremely different seed oil contents were selected for in-depth analysis.
In the present study, there are two main research objectives. One is to gain a comprehensive understanding about evolutionary relationship of the LACS gene family in Arabidopsis, B. napus, B. rapa, and B. oleracea. And the other is to obtain an important clue for further studies on the functions of BnaLACSs in B. napus seed oil accumulation by looking for the BnaLACS genes for differential expression in extremely high oil content (P1-HO) and low oil content (P2-LO) B. napus cultivars.
Materials and Methods
Identification of LACS Proteins in B. napus, B. rapa, and B. oleracea
Sequences of the nine Arabidopsis LACS proteins (AtLACSs) were from the Arabidopsis genome database (https://www.Arabidopsis.org/) (Lamesch et al. 2012). To identify the candidate LACS proteins in B. napus genome, the BLASTP analysis (Altschul et al. 1997) was carried out using AtLACS proteins as query with a threshold e-value of 1e-20. Similarly, LACSs in B. rapa and B. oleracea were identified by BLASTP analysis in the BRAD database (https://brassicadb.org/brad/index.php) as well. The coding sequences (CDSs) and genomic sequences of homologous in B. rapa, B. oleracea, and B. napus were obtained from the BRAD (Cheng et al. 2011).
Phylogenetic Analyses of the LACS Families in B. napus
To comprehensively understand the evolutionary relationships of LACS family members, a phylogenetic tree was generated based on the multiple alignments of the LACS proteins in A. thaliana, B. rapa, B. oleracea, and B. napus by the MEGA7.0 program (Tamura et al. 2011, 2013) using the neighbor-joining (NJ) method. The reliability of the phylogenetic tree was calculated by bootstrap analysis with 1000 replications. Pairwise gap deletion mode was used. The dendrogram was visualized using the program FigTree v1.4.2 (https://tree.bio.ed.ac.uk/software/figtree/) (Zhu et al. 2015).
Protein Properties and Sequence Analyses
The molecular weight (MW) and isoelectric points (pI) of the candidate LACS proteins were predicted by the online ExPASy proteomics server database (https://web.expasy.org/peptide_mass/) (Gasteiger 2003). The protein tertiary structures were predicted using the online SWISS-MODEL workspace (https://swissmodel.expasy.org/interactive) (Arnold et al. 2006). The Gene Structure Display Server 2.0 (https://gsds.cbi.pku.edu.cn/index.php) was used to conduct exon–intron structure analysis (Guo et al. 2007). The MEME program was applied to identify the conserved motifs (https://meme-suite.org/) (Bailey et al. 2006). Subsequently, all identified conserved domains were annotated using InterProScan (https://www.ebi.ac.uk/interpro/search/sequence-search) (Quevillon et al. 2005). The positional information of all BnaLACSs was acquired from B. napus Genome Browser database (https://www.genoscope.cns.fr/brassicanapus/). The location images of BnaLACSs were then drawn by MapChart Software (Voorrips 2002).
Expression Pattern Analysis of BnaLACS Genes
To further characterize the different expression patterns of the BnaLACSs in various organs, we analyzed RNA-sequencing (RNA-seq) data. Transcriptome sequencing datasets were deposited in the BioProject ID PRJNA358784, which includes various B. napus cultivar ZS11 tissues of roots, stems, leaves, buds, anthocaulus, anthers, sepal, filaments, petal, stamens, pistils, the top of main inflorescence flowers, silique pericarps, seeds, seed coat, and embryos at seedling, budding, initial flowering, and full-bloom stages. The expression levels of all BnaLACS genes were quantified based on their fragments per kilobase of exon per million mapped fragments (FPKM) values using Cufflinks with default parameters (Trapnell et al. 2012), and then RNA-seq results were extracted according to their B. napus code.
To further confirm the reliability of RNA-seq, the expressions of BnaLACSs in different tissues at distinct developmental stages were analyzed by qRT-PCR method. Meanwhile, the expression patterns of BnaLACSs in the seed development were also analyzed, using the RNA-seq data in GEO in NCBI (https://www.ncbi.nlm.nih.gov/geo) (accession number GSE77637) (Wan et al. 2016). For RNA-Seq results, log2 expression values of the 34 BnaLACSs were used for heatmap generation using HemI software (Deng et al. 2014). Moreover, 19 BnaLACSs were further selected for comparative analyzing their expressions between the high- (P1-HO) and low oil content (P2-LO) B. napus cultivars.
Plant Materials Used for Gene Expression Analysis
Seeds of B. napus cultivar Zhongshuang 11 (ZS11), SWU47(P1-HO) (approximately 43% oil content in the seeds), and Ningyou12 (P2-LO) (about 33% oil content in the seeds) were provided by Chongqing Engineering Research Center for Rapeseed, China. Plants were cultivated in field conditions at Beibei, Chongqing (29°45′N, 106°22′E, 238.57 m). Each accession contained three rows: 10 plants per row, 30 cm between rows, and 20 cm between plants within each row. All accessions (ZS11, SWU47, and Ningyou12) were sown at the end of September in 2016, and harvested at the beginning of May in the following year. Field fertilization is carried out according to local customs and does not require any chemical treatment. To verify the reliability of RNA-seq in ZS11, tissues of roots (Ro-f), stems (St-f), leaves (Le-f), flowers (Fl-f), buds (Bu-f) in the flowering period; seeds (Se-7d), silique pericarps (SP-7d), seeds (Se-14d), silique pericarps (SP-14d), seeds (Se-21d), silique pericarps (SP-21d), stems (St-30d), seeds (Se-30d), silique pericarps (SP-30d), seeds (Se-40d), and silique pericarps (SP-40d) (7, 14, 21, 30, 40 days after pollination, respectively) were collected. Meanwhile, to survey the expression patterns of BnaLACSs in seeds at different developmental stages of P1-HO and P2-LO, seeds on the main inflorescence after pollination at 7 (Se-7d), 14 (Se-14d), 21(Se-21d), 30 (MSe-30d), 40 (Se-40d) days and seeds on the primary branches at 30 (BSe-30d) days were collected, respectively. For each sample, three biological replicates were collected from three independent plants. All samples were immediately frozen in liquid nitrogen and stored at − 80 ℃ until use.
RNA Isolation and Real-Time Quantitative PCR
Total RNA was extracted from all samples with the EZ-10 DNAaway RNA Mini-prep Kit (Sangon Biotech Co., Ltd, Shanghai, China). Then cDNA was synthesized from 1 µg of total RNA using PrimeScript™ RT reagent Kit (Perfect Real Time; TaKaRa Biotechnology, Dalian, China). Quantitative real-time PCR (qRT-PCR) was performed using SYBR Premix Ex Taq II (Perfect Real Time; TaKaRa, Dalian, China) according to the methods described in Lu et al. (Lu et al. 2015). To gain reliable and reproducible results, we performed three technical replicates for each sample. BnACTIN7 (EV116054) was used as an internal control to evaluate relative gene expression levels (Wu et al. 2010). The values represent the average ± standard error (SE) from the three independent biological replicates. All the qRT-PCR primers are listed in Table S2.
Results
Identification and Phylogenetic Analysis of LACSs in Brassica napus
After conducting the local BLASTN and BLASTP analysis, 34, 12, and 16 LACS genes were obtained from B. napus, B. rapa, and B. oleracea genomes, respectively. The amino acid (aa) residues in length of the putative 34 BnaLACS proteins varied from 129 (BnaLACS9-3) to 960 (BnaLACS3-1), with an average length of 562 aa. The theoretical pI values ranged from 5.11 (BnaLACS1-1) to 9.51 (BnaLACS9-3), including 22 members exhibiting pI values < 7 (Table 1). Furthermore, the theoretical molecular weight (MW) varied from 2546.15 Da (BnaLACS6-6) to 107,784.56 Da (BnaLACS3-1). Similarly, the LACS genes in B. rapa and B. oleracea genomes are listed in Table S1.
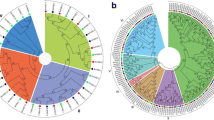
To understand the phylogenetic relationships among LACS proteins from A. thaliana, B. rapa, B. oleracea, and B. napus, we constructed an unrooted neighbor-joining (NJ) phylogenetic tree using MEGA7.0 software (Tamura et al. 2011, 2013) based on the multi-alignment of the full-length amino acid sequences. The generated tree was clustered into four groups, termed A, B, C, and D, based on the topology and bootstrap values of the phylogenetic tree (Fig. 1). AtLACS1, ten BnaLACSs, two BraLACSs, and four BolLACSs belonged to the group A. 24 LACS proteins, including ten BnaLACSs, four AtLACSs, five BraLACSs, and five BolLACSs belonged to the group B. Group C consisted of AtLACS6, AtLACS7, eight BnaLACSs, three BraLACSs, and four BolLACSs. AtLACS8, AtLACS9, six BnaLACSs, two BraLACSs, and three BolLACSs formed Group D. These results indicated that the homologous genes of LACS underwent a big expansion in B. napus as compared with those in Arabidopsis.
The phylogenetic tree of LACS proteins from A. thaliana, B. rapa, B. oleracea, and B. napus. LACSs proteins were used to construct the NJ tree with 1000 bootstraps based on the protein sequences in A. thaliana, B. rapa, B. oleracea, and B. napus using MEGA 7.0 program. The LACS proteins were clustered into four groups
Chromosomal Distribution, Genomic Structure, Conserved Motif, and Tertiary Structure of BnaLACSs
Chromosomal location analysis showed that the 34 BnaLACSs were mapped to 17 of the 19 B. napus chromosomes, and the genes were unevenly distributed on almost all chromosomes except for A08 and C08 chromosomes (Fig. S1). Chromosome A05 contained the largest number (4) of BnaLACSs, chromosomes C03 and C07 contained three genes; chromosomes A02, A03, C04, C05, and C09 contained two BnaLACSs; while only one BnaLACS gene was located on chromosomes A01, A04, A06, A07, A09, A10, C01, C02, and C06, respectively (Fig. S1). In addition, three and two BnaLACS genes are located on Ann_random and Cnn_random, respectively.
Genomic organization plays an important role in the evolution of a gene family (Xu et al. 2012; Wang et al. 2015). To further examine the exon–intron structure of BnaLACSs, the CDSs and their corresponding genomic sequences of BnaLACSs were aligned manually. The number of exons showed a high degree of divergence among the 34 BnaLACSs, ranging from 3 to 25 (Fig. 2a). However, the BnaLACSs within the same group shared similar gene structures, indicating the close relationship. For instance, almost all genes in Group B had 17 to 21 exons, except BnaLACS3-1. Most members (70%) in Group C had 23 exons and genes in Group D had 10 to 11 exons, except BnaLACS9-1 and BnaLACS9-2.
a The exon–intron structure of the AtLACSs and BnaLACSs according to their phylogenetic relationships. An unrooted phylogenetic tree was constructed with the neighbor-joining method (1000 bootstrap replicates) based on the full-length protein sequences of 9 AtLACSs and 34 BnaLACSs. Exon–intron structure analyses of the AtLACSs and BnaLACSs were carried out using the online tool GSDS. The length of the exons and introns of each AtLACS and BnaLACS gene is proportional. b The conserved motifs of the LACS proteins in A. thaliana and B. napus according to their phylogenetic relationships. The conserved motifs were identified by MEME program. Each motif is indicated by a colored box numbered at the bottom and gray lines represent the non-conserved sequences. The length of motifs in each protein is proportional. At: A. thaliana; Bna: B. napus
We predicted 20 putative conserved motifs in all the AtLACS and BnaLACS proteins using the MEME program. As shown in Fig. 2b, motifs 2, 10, and 17 were detected in Group A–C. Motifs 1–9, 12, and 18 were observed in many LACS proteins, accounting for 83.7, 88.4, 88.4, 86, 86, 88.4, 81.4, 88.4, 86, 90.7, and 86% of the total LACS proteins investigated, respectively. Motif 15,16, 19, and 20 was only detected in Group C, A, B, and D, respectively. According to InterProScan annotation, motif 4 was an AMP-binding enzyme C-terminal domain, motifs 1, 2, 6, and 11 were AMP-dependent synthetase/ligase. These five motifs are functionally important for LACS proteins, and the remaining 15 conserved motifs were not annotated in InterProScan yet.
Previous study indicated that tertiary structure and evolution have a weak but recognizable correlation (Choi et al. 2007). To further examine the tertiary structural features of LACS proteins, we predicted the tertiary structures of LACS proteins in A. thaliana and B. napus using the online SWISS-MODEL workspace. As shown in Fig. S2, the tertiary structures of BnaLACS proteins in Group A and B are quite different, which implied a functional divergence of BnaLACSs. However, the tertiary structures of BnaLACSs in Group C and Group D are relatively conservative.
Expression Profiles of BnaLACSs in Various Tissues
To explore the roles of BnaLACSs, the expression patterns of all 34 BnaLACSs in various tissues of B. napus ZS11 at different developmental stages were investigated using RNA-seq dataset (BioProject ID PRJNA358784) (Zhou et al. 2017; Di et al. 2018).
The current study showed that the expression levels of most BnaLACS members differed in various tissues, suggesting the different functions of these genes (Fig. 3 and Table S3). Fourteen BnaLACS genes (BnaLACS1-2, BnaLACS1-3, BnaLACS1-9, BnaLACS1-10, BnaLACS2, BnaLACS4, BnaLACS6-1, BnaLACS6-2, BnaLACS8, BnaLACS9-3, and BnaLACS9-4) were highly expressed in nearly all tissues, which suggested that these genes play a sustainable role across B. napus growth. Conversely, eight BnaLACS genes (BnaLACS1-1, BnaLACS1-6, BnaLACS1-7, BnaLACS1-8, BnaLACS6-5, BnaLACS6-6, BnaLACS9-1, and BnaLACS9-2) were nearly not expressed in all tissues (Fig. 3 and Table S3). The expression abundances of the same gene in the same organs may be varied at different developmental stages, suggesting differences in the temporal expression. Besides, several genes (BnaLACS5-1, 5–2, 5–3, and 5–4) are expressed only in buds, anthers, and stamens, indicating they may be expressed at specific organs. In short, a few BnaLACS genes have a relatively wider range of expression profiles while most of the genes are highly expressed only in individual tissues (Fig. 3 and Table S3).
The expression patterns of BnaLACSs in different tissues or organs at various developmental stages in B. napus. The abbreviations combinations above the heatmap indicate the different tissues or organs at different developmental stages from B. napus ZS11 (listed in Table S5). The heatmap was drawn using Heatmap Illustrator (HemI) (Deng et al. 2014). The bar on the lower right corner represents log2 expression values, and green represents low expression and red stands for high expression
To ensure the reliability of the expression patterns of BnaLACSs based on transcriptomic analysis, we selected 18 BnaLACSs with relative higher expression levels from different groups to analyze their expressions in 16 tissues or organs of ZS11 by qRT-PCR. Our result showed that the expression patterns from qRT-PCR were very similar to that of RNA-seq, which confirmed that the reliability and reproducibility of our study (Figs. 3, 4).
To further understand the roles of BnaLACSs for B. napus seed oil accumulation at seed developmental stage, we further analyzed the expressions of the 34 BnaLACSs at different seed developmental stages (weeks after pollination, WAP), based on previously published RNA-seq data (accession number GSE77637) from GEO in NCBI (https://www.ncbi.nlm.nih.gov/geo) (Wan et al. 2017). We found that eight BnaLACS genes (BnaLACS4-1, 4–2, 6–1, 6–2, 8–1, 8–2, 9–3, and 9–4) were expressed in developmental seeds at the four stages, and six genes (BnaLACS1-2, 1–3, 1–9, 1–10, 2–1, and 2–2) showed relatively higher expressions in seeds at 2 WAP, 4 WAP, and 6 WAP stages. These results suggested the important roles of these genes in seed oil formation in B. napus. BnaLACS7-1 and 7–2 have high transcriptional levels only in seeds at 8 WAP stage, which implied that both genes may play important roles in fatty acid oxidation (Fig. 5).
Tissue expression profiles of selected 18 BnaLACSs at different developmental stages as determined by quantitative real-time PCR (qRT-PCR). The x axis corresponds to the different tissues and organs/developmental stages of B. napus “ZS11″, roots (Ro-f); stems (St-f); leaves (Le-f); flower (Fl-f); bud (Bu-f) in the flowering period; seeds (Se-7d, Se-14d, Se-21d, Se-30d, Se-40d); silique pericarps (SP-7d, SP-14d, SP-21d, SP-30d, SP-40d) after flowering 7, 14, 21, 30, and 40 days, respectively. BnACTIN7 (EV116054) were used as the reference gene (Wu et al. 2010). Values represent the average ± standard error (SE) of three biological replicates with three technical replicates at each developmental stage. Error bars denote SE among three experiments
Spatial-specific expression profiles of BnaLACSs in the process of seed development. The spatial-specific expression levels of 34 BnaLACSs were obtained from the RNA-seq data (accession number GSE77637). This heat map was generated based on the log2 expression values of 34 BnaLACS genes at seed developmental stage (weeks after pollination: WAP): 2 WAP, 4 WAP, 6 WAP, 8 WAP in B. napus. Green indicates low expression and red indicates high expression
Comparative Expression Analysis of BnaLACSs in Two B. napus Cultivars with Different Oil Contents
In Arabidopsis, LACSs can activate free fatty acids into acyl-CoA thioesters, and thus they play a key role in fatty acid metabolism (Shockey et al. 2002). To further explore the roles of BnaLACSs in seed oil accumulation in B. napus, 19 BnaLACSs with relative high expression levels in seeds were further selected to compare their expression patterns in seeds at different developmental stages in two rapeseed cultivars with extremely high-(P1-HO, approximately 43% oil contents in seeds) or low oil content (P2-LO, approximately 33% oil contents in seeds) by qRT-PCR method.
As shown in Fig. 6, the expression levels of BnaLACS1-10, 4–1, 6–4, 9–3, and 9–4 in P1-HO (high oil content) were significantly higher than that of P2-LO (low oil content) in almost all seed tissues investigated. This indicates that these five BnaLACSs may play a vital role in seed oil biosynthesis.
qRT-PCR results of 18 selected BnaLACSs in seeds at different developmental stages between P1-HO (high oil content) and P2-LO (low oil content) Brassica napus. Seeds on the main inflorescence after pollination at 7 (Se-7d), 14 (Se-14d), 21(Se-21d), 30 (MSe-30d), 40 (Se-40d) days and seeds on the primary branches at 30 (BSe-30d) days were used for qRT-PCR analysis. Expression levels of BnaLACSs in P1-HO significantly different to P2-LO are indicated by asterisks (Student’s t test, *: p < 0.05, **: p < 0.01)
Subcellular localization prediction showed that BnaLACS6-4, 9–3 and 9–4 proteins are located at the chloroplast, which is the site of de novo fatty acid synthesis in plants (Schnurr et al. 2002); BnaLACS1-10 and 4–1 are located at the nucleus and cytoplasm, respectively. Therefore, it was suggested that BnaLACS6-4, 9–3, and 9–4 may be involved in fatty acid synthesis in chloroplast, and BnaLACS1-10 and 4–1 may play a vital role in eukaryotic pathway of lipid biosynthesis. The comparative analysis and the subcellular localization prediction result revealed that BnaLACS6-4, BnaLACS9-3, and BnaLACS9-4 are the three main homologues involved in fatty acid synthesis and further seed oil content accumulation in B. napus.
Discussion
To date, various functional research programs of LACS genes were conducted in many plant species, which proved the critical roles of long-chain acyl-coenzyme A (CoA) synthetases (LACSs) in fatty acid metabolism (Shockey et al. 2002; He et al. 2007; Wang and Li 2009; Tan et al. 2014). However, few related works have been applied in B. napus, a widely cultivated oil crop. In this study, we conducted a comprehensive analysis of the LACS gene family in B. napus genome, accompanied by expression assay of the candidate BnaLACSs in various tissues at different stages and between two extremely varieties with different oil contents.
In this study, a total of 12, 16, and 34 LACS genes were identified in the genomes of B. rapa, B. oleracea, and B. napus, respectively. The sum of BraLACSs and BolLACSs is less than the number of BnaLACSs, which hinted that there may be gene redundancies in BnaLACSs. Molecular characterizations showed great variations among the 34 BnaLACSs, where the molecular weights and pIs of them were obviously different among groups; however, members in the same group possessed relatively similar molecular masses and pIs. It was shown that divergences in exon–intron structures can lead to changes of gene function and thus played a crucial role in the evolution of gene families (Xu et al. 2012). In our study, variant exon–intron structures of the BnaLACS genes in different groups revealed the diversification in gene structure. Moreover, we found that BnaLACSs with similar gene structure also have similar tertiary structures and conserved motifs. Notably, the gene structure, tertiary structures, and conserved motifs of BnaLACS1-1, 6–5, 6–6, 9–1, and 9–2 are markedly different from those of the remaining BnaLACSs; consistent with this trend, these genes are hardly expressed in all tissues examined. Given some gene fragments in these genes were lost, it was suggested these fragments deletion may lead to loss of gene function.
Expression analyses have revealed that LACS genes have different expression patterns in various tissues in Arabidopsis, rice, cotton, and soybean (Fulda et al. 2002; Shockey et al. 2002; Ichihara et al. 2003; Wang and Li 2009). In Arabidopsis, most LACS genes have quite different expression levels in developing seeds; LACS1, LACS2, LACS4, and LACS9 showed relative higher expression levels compared with LACS6, LACS7, and LACS8, and LACS3 and LACS5 are not expressed in the developing seeds (Shockey et al. 2002). In the current study, BnaLACSs also exhibited differential expression patterns in different tissues. Notably, BnaLACS4-1, 4–2, 6–1, 6–2, 8–1, 8–2, 9–3, and 9–4 were highly expressed in all tissues (Fig. 3), which is similar with their counterparts in Arabidopsis (Shockey et al. 2002). Similarly, some BnaLACSs, such as 5–1, 5–2, 5–3, and 5–4, were specifically expressed in buds, anthers, and stamens; accordingly, their homologous gene AtLACS5 is specifically expressed in flowers (Shockey et al. 2002). And it was demonstrated that the AtLACS4 homologous gene in B. napus, BnaLACS4, resided at endoplasmic reticulum (ER) and was involved in lipid biosynthesis as well (Tan et al. 2014). These results suggested that the functions of the LACS genes in organs of different species are somewhat conserved. Consequently, we speculated that these genes may have important functions in the process of lipid metabolism, just like the homolog genes in Arabidopsis (Zhao et al. 2010).
On the basis of the above RNA-Seq analyses, we intuitively observed that BnaLACS1 (1–2, 1–3, 1–4, 1–9, 1–10), BnaLACS2 (2–1, 2–2), BnaLACS4 (4–1, 4–2), BnaLACS6 (6–1, 6–2, 6–4), BnaLACS7 (7–1, 7–2), BnaLACS8 (8–1, 8–2), and BnaLACS9 (9–3, 9–4) exhibited higher expression levels during seed development. Subsequently, we further analyzed these 18 BnaLACSs to compare their expressions in seeds between high oil content (P1-HO) and low oil content (P2-LO) B. napus cultivars. The results showed that the expression levels of BnaLACS1-10, 4–1, 6–4, 9–3, and 9–4 in P1-HO were significantly higher than that of P2-LO, which indicated that these genes may play a vital role in seed oil biosynthesis and high oil content formation in B. napus. Interestingly, we found two AtLACS4 homologs, BnaLACS4-1 and 4–2, were highly conserved with 99.7% amino acid similarity (only have two different amino acids), but their expression patterns are quite different between high (P1-HO) and low oil (P2-LO) (Fig. 5). It was verified that BnLACS4 (BnaLACS4-2 in this study) was highly homologous to AtLACS4 and could also complement a LACS-deficient yeast strain YB525 and even can increase the lipid content in the yeast strain pep4 by overexpression BnLACS4(Tan et al. 2014). The present study showed that the expression level of BnaLACS4-1 in P1-HO was significantly higher than that of P2-LO, as compared to BnaLACS4-2. Moreover, BnaLACS4-1 were successfully located within the QTL confidence interval associated with oil content (Chao et al. 2017). Based on these results, though the expressions of BnaLACS4-1 and 4–2 were different that might due to the expression diversification occurred in the promoter regions of them, it was speculated that the BnaLACS4s homolog genes have important role in B. napus lipid biosynthesis as well. And BnaLACS4-1 may play a relatively more important role in lipid biosynthesis and contribute to the formation of high oil content. Besides, the expression level of BnaLACS1-10, 6–4, 9–3, and 9–4 in P1-HO was also significantly higher than that of P2-LO, indicating their important roles in seed fatty acids biosynthesis and high oil content formation in B. napus. Taken together, the present study characterized the difference expressions of BnaLACSs during seed oil accumulation process, based on a comprehensive survey of phylogenetic relationships, gene structures, conserved motifs, and expression profiles of the LACS genes in different tissues of B. napus. And the comparative analysis of BnaLACSs in two B. napus cultivars with different seed oil contents revealed that BnaLACS1-10, 4–1, 6–4, 9–3, and 9–4 genes may play important roles for long-chain fatty acid synthesis and further seed oil accumulation in B. napus. In the next study, we will further research the molecular functions of BnaLACS1-10, 4–1, 6–4, 9–3, and 9–4 genes and explore the application potential of these genes in improving the seed oil content of B. napus. In general, the current study provides a foundation in further understanding the characterization of LACS genes family and for molecular breeding studies to increase the seed oil content and quality in B. napus.
References
Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201
Bailey TL, Williams N, Misleh C, Li WW (2006) MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res 34:W369–373
Black PN, DiRusso CC (2007) Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation. Bba-Mol Cell Biol L 1771:286–298
Black PN, Dirusso CC, Metzger AK, Heimert TL (1992) Cloning, sequencing, and expression of the fadd gene of Escherichia-Coli encoding acyl coenzyme-a synthetase. J Biol Chem 267:25513–25520
Chalhoub B, Denoeud F, Liu SY, Parkin IAP, Tang HB, Wang XY, Chiquet J, Belcram H, Tong CB, Samans B, Correa M, Da Silva C, Just J, Falentin C, Koh CS, Le Clainche I, Bernard M, Bento P, Noel B, Labadie K, Alberti A, Charles M, Arnaud D, Guo H, Daviaud C, Alamery S, Jabbari K, Zhao MX, Edger PP, Chelaifa H, Tack D, Lassalle G, Mestiri I, Schnel N, Le Paslier MC, Fan GY, Renault V, Bayer PE, Golicz AA, Manoli S, Lee TH, Thi VHD, Chalabi S, Hu Q, Fan CC, Tollenaere R, Lu YH, Battail C, Shen JX, Sidebottom CHD, Wang XF, Canaguier A, Chauveau A, Berard A, Deniot G, Guan M, Liu ZS, Sun FM, Lim YP, Lyons E, Town CD, Bancroft I, Wang XW, Meng JL, Ma JX, Pires JC, King GJ, Brunel D, Delourme R, Renard M, Aury JM, Adams KL, Batley J, Snowdon RJ, Tost J, Edwards D, Zhou YM, Hua W, Sharpe AG, Paterson AH, Guan CY, Wincker P (2014) Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 345:950–953
Chao H, Wang H, Wang X, Guo L, Gu J, Zhao W, Li B, Chen D, Raboanatahiry N, Li M (2017) Genetic dissection of seed oil and protein content and identification of networks associated with oil content in Brassica napus. Sci Rep 7:46295
Cheng F, Liu SY, Wu J, Fang L, Sun SL, Liu B, Li PX, Hua W, Wang XW (2011) BRAD, the genetics and genomics database for Brassica plants. Bmc Plant Biol 11:136
Choi SC, Hobolth A, Robinson DM, Kishino H, Thorne JL (2007) Quantifying the impact of protein tertiary structure on molecular evolution. Mol Biol Evol 24:1769–1782
Deng W, Wang Y, Liu Z, Cheng H, Xue Y (2014) HemI: a toolkit for illustrating heatmaps. PLoS ONE 9:e111988
Di F, Jian H, Wang T, Chen X, Ding Y, Du H, Lu K, Li J, Liu L (2018) Genome-wide analysis of the PYL gene family and identification of PYL genes that respond to abiotic stress in Brassica napus. Genes (Basel) 9:156
Fulda M, Shockey J, Werber M, Wolter FP, Heinz E (2002) Two long-chain acyl-CoA synthetases from Arabidopsis thaliana involved in peroxisomal fatty acid beta-oxidation. Plant J 32:93–103
Gasteiger E (2003) ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31:3784–3788
Groot PH, Scholte HR, Hulsmann WC (1976) Fatty acid activation: specificity, localization, and function. Adv Lipid Res 14:75–126
Guo AY, Zhu QH, Chen X, Luo JC (2007) GSDS: a gene structure display server. Hereditas 29:1023–1026
He X, Chen GQ, Kang ST, McKeon TA (2007) Ricinus communis contains an acyl-CoA synthetase that preferentially activates ricinoleate to its CoA thioester. Lipids 42:931–938
Ichihara K, Kobayashi N, Saito K (2003) Lipid synthesis and acyl-CoA synthetase in developing rice seeds. Lipids 38:881–884
Jang YE, Kim MY, Shim S, Lee J, Lee S-H (2015) Gene expression profiling for seed protein and oil synthesis during early seed development in soybean. Genes Genom 37:409–418
Jessen D, Roth C, Wiermer M, Fulda M (2015) Two activities of long-chain acyl-coenzyme A synthetase are involved in lipid trafficking between the endoplasmic reticulum and the plastid in Arabidopsis. Plant Physiol 167:351–366
Jetter R, Kunst L (2008) Plant surface lipid biosynthetic pathways and their utility for metabolic engineering of waxes and hydrocarbon biofuels. Plant J 54:670–683
Jia B, Song Y, Wu M, Lin B, Xiao K, Hu Z, Huang Y (2016) Characterization of long-chain acyl-CoA synthetases which stimulate secretion of fatty acids in green algae Chlamydomonas reinhardtii. Biotechnol Biofuels 9:184
Jian H, Lu K, Yang B, Wang T, Zhang L, Zhang A, Wang J, Liu L, Qu C, Li J (2016) Genome-wide analysis and expression profiling of the SUC and SWEET gene families of sucrose transporters in oilseed rape (Brassica napus L.). Front Plant Sci 7:1464
Lamesch P, Berardini TZ, Li D, Swarbreck D, Wilks C, Sasidharan R, Muller R, Dreher K, Alexander DL, Garcia-Hernandez M, Karthikeyan AS, Lee CH, Nelson WD, Ploetz L, Singh S, Wensel A, Huala E (2012) The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res 40:D1202–1210
Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, Debono A, Durrett TP, Franke RB, Graham IA, Katayama K, Kelly AA, Larson T, Markham JE, Miquel M, Molina I, Nishida I, Rowland O, Samuels L, Schmid KM, Wada H, Welti R, Xu C, Zallot R, Ohlrogge J (2010) Acyl-lipid metabolism. Arabidopsis Book 8:e0133
Lu S, Song T, Kosma DK, Parsons EP, Rowland O, Jenks MA (2009) Arabidopsis CER8 encodes long-chain acyl-coa synthetase 1 (LACS1) that has overlapping functions with LACS2 in plant wax and cutin synthesis. Plant J 59:553–564
Lu K, Guo W, Lu J, Yu H, Qu C, Tang Z, Li J, Chai Y, Liang Y (2015) Genome-wide survey and expression profile analysis of the mitogen-activated protein kinase (MAPK) gene family in Brassica rapa. PLoS ONE 10:e0132051
Overath P, Pauli G, Schairer HU (1969) Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur J Biochem 7:559–574
Pongdontri P, Hills M (2001) Characterization of a novel plant acyl-CoA synthetase that is expressed in lipogenic tissues of Brassica napus L. Plant Mol Biol 47:717–726
Pulsifer IP, Kluge S, Rowland O (2012) Arabidopsis long-chain acyl-CoA synthetase 1 (LACS1), LACS2, and LACS3 facilitate fatty acid uptake in yeast. Plant Physiol Biochem 51:31–39
Quevillon E, Silventoinen V, Pillai S, Harte N, Mulder N, Apweiler R, Lopez R (2005) InterProScan: protein domains identifier. Nucleic Acids Res 33:W116–W120
Schnurr JA, Shockey JM, de Boer GJ, Browse JA (2002) Fatty acid export from the chloroplast. Molecular characterization of a major plastidial acyl-coenzyme A synthetase from Arabidopsis. Plant Physiol 129:1700–1709
Schnurr J, Shockey J, Browse J (2004) The acyl-CoA synthetase encoded by LACS2 is essential for normal cuticle development in Arabidopsis. Plant Cell 16:629–642
Shockey JM, Fulda MS, Browse JA (2002) Arabidopsis contains nine long-chain acyl-coenzyme a synthetase genes that participate in fatty acid and glycerolipid metabolism. Plant Physiol 129:1710–1722
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tan X, Zheng X, Zhang Z, Wang Z, Xia H, Lu C, Gu S (2014) Long chain acyl-coenzyme a synthetase 4 (BnLACS4) gene from Brassica napus enhances the yeast lipid contents. J Integr Agric 13:54–62
Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7:562–578
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78
Wan H, Cui Y, Ding Y, Mei J, Dong H, Zhang W, Wu S, Liang Y, Zhang C, Li J, Xiong Q, Qian W (2016) Time-series analyses of transcriptomes and proteomes reveal molecular networks underlying oil accumulation in Canola. Front Plant Sci 7:2007
Wan HF, Cui YX, Ding YJ, Mei JQ, Dong HL, Zhang WX, Wu SQ, Liang Y, Zhang CY, Li JN, Xiong Q, Qian W (2017) Time-series analyses of transcriptomes and proteomes reveal molecular networks underlying oil accumulation in Canola. Front Plant Sci 7:1
Wang XL, Li XB (2009) The GhACS1 gene encodes an acyl-CoA synthetase which is essential for normal microsporogenesis in early anther development of cotton. Plant J 57:473–486
Wang L, Yin X, Cheng C, Wang H, Guo R, Xu X, Zhao J, Zheng Y, Wang X (2015) Evolutionary and expression analysis of a MADS-box gene superfamily involved in ovule development of seeded and seedless grapevines. Mol Genet Genomics 290:825–846
Weng H, Molina I, Shockey J, Browse J (2010) Organ fusion and defective cuticle function in a lacs1 lacs2 double mutant of Arabidopsis. Planta 231:1089–1100
Wu G, Zhang L, Wu Y, Cao Y, Lu C (2010) Comparison of five endogenous reference genes for specific PCR detection and quantification of Brassica napus. J Agric Food Chem 58:2812–2817
Xu G, Guo C, Shan H, Kong H (2012) Divergence of duplicate genes in exon-intron structure. Proc Natl Acad Sci USA 109:1187–1192
Zhao L, Katavic V, Li F, Haughn GW, Kunst L (2010) Insertional mutant analysis reveals that long-chain acyl-CoA synthetase 1 (LACS1), but not LACS8, functionally overlaps with LACS9 in Arabidopsis seed oil biosynthesis. Plant J 64:1048–1058
Zhou Y, Xu D, Jia L, Huang X, Ma G, Wang S, Zhu M, Zhang A, Guan M, Lu K, Xu X, Wang R, Li J, Qu C (2017) Genome-wide identification and structural analysis of bZIP transcription factor genes in Brassica napus. Genes (Basel) 8:E288
Zhu X, Dunand C, Snedden W, Galaud JP (2015) CaM and CML emergence in the green lineage. Trends Plant Sci 20:483–489
Acknowledgements
This study was funded by National Key R & D Program of China (2018YFD0200903; 2018YFD0100504), the “111″ Project (B12006), Modern Agro-industry Technology Research System (CARS-13), the National Natural Science Foundation of China (31400063; 31571701, U1302266), Chongqing Minsheng Technology Funds (cstc2016shms-ztzx80020), and Fundamental Research Funds for the Central Universities (XDJK2017B030). We extend our thanks to the reviewers for their careful reading and helpful comments on this manuscript.
Author information
Authors and Affiliations
Contributions
J.N.L. and N.N.L. conceived and designed the experiments. Z.C.X. performed the experiments. Z.C.X., S. F.W., J.J.S., L.Y. Z., C.Z., H.Y., H.Y.Z., B.Y., L.J.W., H.D., C.M.Q, and K.L. analyzed the data. Z.C.X. and N.N.L. wrote the paper. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10528_2019_9921_MOESM1_ESM.tif
Chromosomal locations of the BnaLACS genes in the B. napus genome. The distribution of each BnaLACS gene was mapped according to the B. napus genome. The chromosome numbers are indicated at the top of each chromosome. The gene names are on the right side of each chromosome, according to the physical location (left side) of each BnaLACS gene. Supplementary file1 (XLSX 13 kb)
10528_2019_9921_MOESM2_ESM.tif
3D models of identified LACSs proteins. The tertiary structures were generated by online SWISS-MODEL Workspace. Supplementary file2 (XLSX 13 kb)
Rights and permissions
About this article
Cite this article
Xiao, Z., Li, N., Wang, S. et al. Genome-Wide Identification and Comparative Expression Profile Analysis of the Long-Chain Acyl-CoA synthetase (LACS) Gene Family in Two Different Oil Content Cultivars of Brassica napus. Biochem Genet 57, 781–800 (2019). https://doi.org/10.1007/s10528-019-09921-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-019-09921-5