Abstract
Plasminogen activator inhibitor type 1 (PAI-1) is a serine protease inhibitor (Serpine 1), and it inhibits both tissue plasminogen activator and urokinase plasminogen activator which are important in fibrinolysis. We aimed to find whether there is a possible association between PAI-1 level, PAI-1 4G/5G polymorphism, and endometrial cancer. PAI-1 levels in peripheral blood were determined in 82 patients with endometrial carcinoma and 76 female healthy controls using an enzyme-linked immunoassay (ELISA). Then, the genomic DNA was extracted and screened by reverse hybridization procedure (Strip assay) to detect PAI 1 4G/5G polymorphism. The levels of PAI-1 in the patients were higher statistically in comparison to controls (P < 0.001). The distribution of PAI-1 4G/5G polymorphism was quite different between patients and controls (P = 0.008), and 4G allelic frequency was significantly higher in the patients of endometrial cancer than in controls (P = 0.026). We found significant difference between Grade 1 and Grade 2+3 patients in terms of the PAI-1 levels (P = 0.047). There was no association between PAI-1 4G/5G polymorphism and the grades of endometrial cancer (P = 0.993). Our data suggest that the level of PAI-1 and PAI-1 4G/5G gene polymorphism are effective in the formation of endometrial cancer. PAI-1 levels are also associated with the grades of endometrial cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fibrinolysis is crucial in the prevention of thrombus, and it also restores blood flow after thrombotic occlusions (Wu and Zhao 2002). Plasminogen which is pivotal regulator in fibrinolysis can be converted into plasmin by tissue plasminogen activator (t-PA) and urokinase plasminogen activator (u-PA) (Dejouvencel et al. 2010). Plasmin is a serine protease involved in intravascular dissolution of fibrin clots (Al-Horani et al. 2015). Plasminogen activator inhibitor-1 (PAI-1) is a member of the serine protease inhibitor (Serpine 1) superfamily, and it is an important factor in the regulation of endogenous fibrinolysis by inhibiting both t-PA and u-PA in the fibrinolytic system (Nikolopoulos et al. 2014). PAI-1 has an inhibitory effect on the fibrinolytic system, and high levels of PAI-1 are associated with thrombosis and cardiovascular diseases (Han et al. 2016). On the other hand, PAI-1 deficiency can cause important hemorrhage. For these reasons, PAI-1 is a regulator of hemostatic processes (Hosokawa et al. 2016).
PAI-1 gene is located on chromosome 7 (7q21.3–q22). There is a common polymorphism known as 4G/5G in the promoter region (Guanosine insertion/deletion gene polymorphism) of this gene. This polymorphism that results in a sequence of 4G instead of 5G in the promoter region of the gene is associated with a small increase in the risk of venous thromboembolism (Said et al. 2012). Homozygosity for the deletion allele (4G/4G) has been associated with higher PAI-1 concentrations than the insertion genotype (5G/5G), and reduced fibrinolytic activity (Dossenbach-Glaninger et al. 2003). Different studies suggest that the level of PAI-1 or PAI-1 4G/5G polymorphism is associated with the formation and prognosis of some cancers including breast cancer, hepatocellular carcinoma, and endometrial cancer (Rabi et al. 2015; Divella et al. 2015; Gilabert-Estellés et al. 2012). PAI-1 may play a critical role in cancer invasion and metastasis (Gouri et al. 2016).
Endometrial cancer is the common disease of the endometrium, and it is a major problem for women’s health. The level of PAI-1 may be an important marker for the prognosis of endometrial cancer. Taponeco et al. demonstrated that there is a progressive increase in the expression of PAI-1 in endometrial carcinoma (Taponeco et al. 2001). Xu et al. declared that the PAI-1 4G/5G polymorphism may contribute to cancer susceptibility and the 4G allele of PAI-1 may be associated with an increased risk of endometrial cancer (Xu et al. 2012).
On the basis of these data, we investigated the association between the level of PAI-1, PAI-1 4G/5G polymorphism, and the formation of endometrial cancer and tumor grades.
Materials and Methods
Study Population
Our study was approved by the Ethics Committee in Cumhuriyet University, Sivas, Turkey. 82 patients who were diagnosed as endometrial cancer in our obstetrics and gynecology department in 2014 and 76 female healthy controls were included in this study. The exact diagnoses of the patients were performed histopathologically. All patients underwent total hysterectomy with bilateral salpingo-oophorectomy. The patients were classified in terms of the tumor grades. Well-differentiated or poorly differentiated tumors were detected under the microscope. Individuals who had alcohol story, obesity problem, and smokers were excluded. The age and body mass index (BMI) of the patients and control group were recorded. PAI-1 measurements were performed in serum samples from venous blood specimens.
Laboratory Analysis
Cytosolic concentrations of PAI-1 were determined by enzyme-linked immunoassay (ELISA) using Human PAI-1 ELISA Kit (Boster Biological Pleasanton, CA) on a Triturus Analyzer (Diagnostics Grifols, Spain).
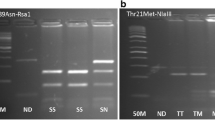
The genomic DNA was extracted from the peripheral blood using DNA isolation kit (Invitek Invisorb Spin Blood Kit, Germany). Relevant gene sequences were amplified in a single (multiplex) amplification reaction, and they were screened for PAI-1 4G/5G polymorphism using reverse hybridization strip assay procedure (Vienna Lab, CVD Strip Assay, GMBH, Austria). Amplification conditions were as follows: pre-PCR, 2 min at 95 °C; denaturation, 15 s at 95 °C; annealing 30 s at 56 °C; extension 30 s at 72 °C, and final extension 3 min at 72 °C. The amplification products were hybridized to a test strip, which contains allele-specific oligonucleotide probes. Biotinylated sequences were detected using streptavidin–alkaline phosphatase and color substrates. Hybridization was performed in an automated incubator (Auto-LIPA, _Innogenetics).
Statistical Analysis
Statistical analysis was performed using SPSS 15.0 program (SPSS Inc., Chicago, IL, USA). Student’s t test was performed for the comparison of BMI and mean age. χ 2 test was used to evaluate the frequencies of alleles and genotypes. The odds ratios were calculated at 95% confidence interval for the association between alleles and risk in the formation of endometrial cancer. Independent-Samples t-Test was used to compare PAI-1 concentrations between groups. P value < 0.05 was considered statistically significant.
Results
Eighty-two patients diagnosed with endometrial cancer and seventy-six female controls were screened in terms of PAI-1 level and PAI-1 4G/5G polymorphism. The mean age and BMI of the patients were not statistically different than controls. The characteristics of the patients and the control group are shown in Table 1. We detected the level of PAI-1 in the patients and controls. Mean PAI-1 levels of the patients and controls were 14.88 ± 2.52 and 12.08 ± 2.01, respectively. There was a significant difference between PAI-1 concentrations of the patients and controls (P < 0.001) (Table 2). PAI-1 levels of the patients were also analyzed for the grades of the disease. In this respect, we divided all patients into two groups. The first group of patients was Grade 1 (n = 56). The second was Grade 2+3 (n = 26). Mean PAI-1 level of Grade 1 patients was 14.50 ± 2.40 and the mean PAI-1 level of Grade 2+3 was 15.69 ± 2.63. We found statistically significant difference between Grade 1 and Grade 2+3 patients in terms of the concentrations of PAI-1 (P = 0.047) (Table 3).
We investigated the distribution of PAI-1 4G/5G polymorphism in the patients and controls. In the patients, the frequency of 4G/4G genotype was 26.8% and 4G allelic frequency was 57.9%. The frequency of 4G/4G genotype and 4G allelic frequency in controls were 14.5 and 42.8%, respectively. The difference between patients and controls was significant in terms of the distribution of PAI-1 4G/5G polymorphism and 4G allelic frequency (P = 0.008 and P = 0.026). PAI-1 4G/4G genotype and 4G allelic frequency were higher in the patients of endometrial cancer than in controls (Table 4).
We also analyzed this polymorphism in Grade 1 and Grade 2+3 patients. The frequencies of 4G/4G genotype and 4G allelic frequency were 26.8 and 58% in Grade 1 patients and 27 and 57.7% in Grade 2+3 patients, respectively. There was no significant difference between Grade 1 and Grade 2+3 cases regarding the distribution of PAI-1 4G/5G polymorphism and 4G allelic frequency (P = 0.993 and P = 0.967). The frequency of 4G and 5G allele was similar in both the groups (Table 5).
Discussion
In this study, we evaluated the effect of PAI-1 level and PAI-1 4G/5G polymorphism on endometrial cancer. It is one of the rare studies analyzing both the PAI-1 4G/5G polymorphism and PAI-1 concentrations in the patients with endometrial cancer.
Plasminogen is a single 90 kDa polypeptide that can be cleaved by plasminogen activators to form plasmin (Drinane et al. 2006). Plasmin defects may cause thrombosis and clots cannot be dissolved entirely. PAI-1 reduces plasminogen activity and causes poor fibrinolysis (Liguori et al. 2014). Plasma PAI-1 levels are affected by a lot of conditions, including body mass, lipid metabolism, glucose concentration, renin–angiotensin–aldosterone system, and genetic variability (Abd El-Aziz and Rezk 2015). There are a lot of studies claiming that PAI-1 level and PAI-1 4G/5G polymorphism are associated with the formation of various tumors and poor prognosis of cancer.
Endometrial cancer is the second most common gynecological cancer in the world (Meyer et al. 2015). These cancers arise from the inner lining of the uterus. Despite the high incidence of endometrial cancer, some features of its management are still unclear (Bogani et al. 2014). Fredstorp-Lidebring et al. claimed that PAI-1 is a prognostic factor in early stage of endometrial cancer (Fredstorp-Lidebring et al. 2001). It is thought that high levels of PAI-1 in endometrial cancer are associated with the poor prognosis of the disease (Gilabert-Estellés et al. 2012). A progressive increase in expression of u-PA and PAI-1 was detected in endometrial carcinoma, and, consequently, PAI-1 may contribute the invasiveness and unfavorable prognosis of endometrial carcinoma independent of other clinicopathological parameters (Taponeco et al. 2001).
On the other hand, it was asserted that very high concentrations of PAI-1 may inhibit angiogenesis and tumor growth as a paradox (Wyganowska-Świątkowska and Jankun 2015). PAI-1 4G/5G polymorphism affects the level of PAI-1, and this polymorphism may contribute to the increase of cancer risk and tumor severity (Xu et al. 2012; Castelló et al. 2006). In a meta-analysis, Wang et al. suggested that the PAI-1 4G/5G polymorphism is associated with increased cancer risk (Wang et al. 2013). Individuals who have 4G/4G genotype may have higher risk of endometrial cancer than the others (Su et al. 2011).
Our study revealed that the levels of PAI-1 were higher in the patients with endometrial carcinoma in comparison to controls. These data support the idea that PAI-1 is effective in cancer formation. Another possibility is the upregulating of PAI-1 in the presence of endometrial cancer. PAI-1 is associated with cell migration and cell proliferation, and it may modulate the course of tumor development and invasion as in angiogenesis and fibrosis (McMahon and Kwaan 2015). Therefore, the level of PAI-1 is important in cancers. PAI-1 may affect the formation of endometrial cancer, but the relationship between the differentiation of this tumor and the level of PAI-1 is uncertain. In a study by Köhler et al., it was found that the median level of PAI-1 was significantly higher in Grade 2 and Grade 3 endometrial cancer patients compared to Grade 1 (Köhler et al. 1997). We also detected higher levels of PAI-1 in Grade 2+3 endometrial cancer patients than in Grade 1. In other words, high-grade cancers had higher PAI-1 levels. Therefore, we thought that PAI-1 may play an important role in cancer progression through the differentiation of endometrial cancer. Our study showed that PAI-1 4G/5G polymorphism may be associated with the formation of endometrial cancer. The frequency of the PAI-1 4G/4G genotype and 4G allelic frequency were significantly higher in the patients than in controls. We could not detect any association between PAI-1 4G/5G polymorphism and the grades of endometrial cancer.
Based on this study, it can be thought that the level of PAI-1 and PAI-1 4G/5G polymorphism are effective in the formation of endometrial cancer, and PAI-1 level is associated with the grade of this tumor. If it consists of a common idea about the effect of PAI-1 levels and 4G/5G polymorphism on endometrial cancer, PAI-1 may be a therapeutic target in the future.
References
Abd El-Aziz TA, Rezk NA (2015) Relation of PAI-1 and TPA genes polymorphisms to acute myocardial infarction and its outcomes in Egyptian patients. Cell Biochem Biophys 71:227–234
Al-Horani RA, Karuturi R, White DT, Desai UR (2015) Plasmin regulation through allosteric, sulfated, small molecules. Molecules 20:608–624
Bogani G, Dowdy SC, Cliby WA, Ghezzi F, Diego Rossetti D, Mariani A (2014) Role of pelvic and para-aortic lymphadenectomy in endometrial cancer: current evidence. J Obstet Gynaecol Res 40:301–311
Castelló R, España F, Vázquez C, Fuster C, Almenar SM, Aznar J et al (2006) Plasminogen activator inhibitor-1 4G/5G polymorphism in breast cancer patients and its association with tissue PAI-1 levels and tumor severity. Thromb Res 117:487–492
Dejouvencel T, Doeuvre L, Lacroix R, Plawinski L, Dignat-George F, Lijnen HR et al (2010) Fibrinolytic cross-talk: a new mechanism for plasmin formation. Blood 115:2048–2056
Divella R, Daniele A, Abbate I, Savino E, Casamassima P, Sciortino G et al (2015) Circulating levels of PAI-1 and SERPINE1 4G/4G polymorphism are predictive of poor prognosis in HCC patients undergoing TACE. Transl Oncol 8:273–278
Dossenbach-Glaninger A, van Trotsenburg M, Dossenbach M, Oberkanins C, Moritz A, Krugluger W et al (2003) Plasminogen activator inhibitor 1 4G/5G polymorphism and coagulation factor XIII Val34Leu polymorphism: impaired fibrinolysis and early pregnancy loss. Clin Chem 49:1081–1086
Drinane MC, Sherman JA, Hall AE, Simons M, Mulligan-Kehoe MJ (2006) Plasminogen and plasmin activity in patients with coronary artery disease. J Thromb Haemost 4:1288–1295
Fredstorp-Lidebring M, Bendahl PO, Brünner N, Casslén B, Högberg T, Långström-Einarsson E et al (2001) Urokinase plasminogen activator and its inhibitor, PAI-1, in association with progression-free survival in early stage endometrial cancer. Eur J Cancer 37:2339–2348
Gilabert-Estellés J, Ramón LA, Braza-Boïls A, Gilabert J, Chirivella M, España F et al (2012) Plasminogen activator inhibitor-1 (PAI-1) 4 G/5 G polymorphism and endometrial cancer. Influence of PAI-1 polymorphism on tissue PAI-1 antigen and mRNA expression and tumor severity. Thromb Res 130:242–247
Gouri A, Dekaken A, El Bairi K, Aissaoui A, Laabed N, Chefrour M, Ciccolini J, Milano G, Benharkat S (2016) Plasminogen activator system and breast cancer: potential role in therapy decision making and precision medicine. Biomark Insights 11:105–111
Han T, Zhang G, Yan D, Yang H, Ma T, Ye Z (2016) Modulation of plasminogen activator inhibitor-1 (PAI-1) by the naphthoquinone shikonin. Fitoterapia 113:117–122
Hosokawa K, Ohnishi-Wada T, Sameshima-Kaneko H, Nagasato T, Miura N, Kikuchi K, Koide T, Maruyama I, Urano T (2016) Plasminogen activator inhibitor type 1 in platelets induces thrombogenicity by increasing thrombolysis resistance under shear stress in an in vitro flow chamber model. Thromb Res 146:69–75
Köhler U, Hiller K, Martin R, Langanke D, Naumann G, Bilek K et al (1997) Tumor-associated proteolytic factors uPA and PAI-1 in endometrial carcinoma. Gynecol Oncol 66:268–274
Liguori R, Quaranta S, Di Fiore R, Elce A, Castaldo G, Amato F (2014) A novel polymorphism in the PAI-1 gene promoter enhances gene expression. a novel pro-thrombotic risk factor? Thromb Res 134:1229–1233
McMahon BJ, Kwaan HC (2015) Components of the plasminogen-plasmin system as biologic markers for cancer. Adv Exp Med Biol 867:145–156
Meyer LA, Bohlke K, Powell MA, Fader AN, Franklin GE, Lee LJ et al (2015) Postoperative radiation therapy for endometrial cancer: American society of clinical oncology clinical practice guideline endorsement of the american society for radiation oncology evidence-based guideline. J Clin Oncol 33:2908–2913
Nikolopoulos GK, Bagos PG, Tsangaris I, Tsiara CG, Kopterides P, Vaiopoulos A et al (2014) The association between plasminogen activator inhibitor type 1 (PAI-1) levels, PAI-1 4G/5G polymorphism, and myocardial infarction: a Mendelian randomization meta-analysis. Clin Chem Lab Med 52:937–950
Rabi ZA, Todorović-Raković N, Vujasinović T, Milovanović J, Nikolić-Vukosavljević D (2015) Markers of progression and invasion in short term follow up of untreated breast cancer patients. Cancer Biomark 15:745–754
Said JM, Tsui R, Borg AJ, Higgins JR, Moses EK, Walker SP et al (2012) The PAI-1 4G/5G polymorphism is not associated with an increased risk of adverse pregnancy outcome in asymptomatic nulliparous women. J Thromb Haemost 10:881–886
Su CK, Yeh KT, Yeh CB, Wang PH, Ho ES, Chou MC et al (2011) Genetic polymorphism of the plasminogen activator inhibitor-1 is associated with an increased risk of endometrial cancer. J Surg Oncol 104:755–759
Taponeco F, Curcio C, Giuntini A, Nardini V, Fornaciari G, Artini PG et al (2001) Expression and prognostic significance of urokinase and plasminogen activator inhibitor type-1 in endometrial hyperplasia and cancer. J Exp Clin Cancer Res 20:239–246
Wang S, Cao Q, Wang X, Li B, Tang M, Yuan W et al (2013) PAI-1 4G/5G polymorphism contributes to cancer susceptibility: evidence from meta-analysis. PLoS ONE 8:e56797
Wu Q, Zhao Z (2002) Inhibition of PAI-1: a new anti-thrombotic approach. Current Drug Targets Cardiovasc Haematol Disord 2:27–42
Wyganowska-Świątkowska M, Jankun J (2015) Plasminogen activation system in oral cancer: relevance in prognosis and therapy (review). Int J Oncol 47:16–24
Xu X, Xie Y, Lin Y, Xu X, Zhu Y, Mao Y et al (2012) PAI-1 promoter 4G/5G polymorphism (rs1799768) contributes to tumor susceptibility: evidence from meta-analysis. Exp Ther Med 4:1127–1133
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yıldırım, M.E., Karakuş, S., Kurtulgan, H.K. et al. The Association of Plasminogen Activator Inhibitor Type 1 (PAI-1) Level and PAI-1 4G/5G Gene Polymorphism with the Formation and the Grade of Endometrial Cancer. Biochem Genet 55, 314–321 (2017). https://doi.org/10.1007/s10528-017-9796-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-017-9796-7