Abstract
RAPD markers were used to detect genetic diversity and population genetic differentiation of Hippophae rhamnoides ssp. yunnanensis, a sea buckthorn endemic to the Qinghai-Tibet plateau. The genetic parameters of percentage of polymorphic bands (92.86%), Nei’s gene diversity (h, 0.255), and Shannon’s index (I, 0.397) indicated high genetic diversity in this subspecies. The subpopulation differentiation suggested that 45.9% of genetic variation was among populations. High genetic differentiation among populations was also detected using AMOVA (47.02%). The main factors responsible for high genetic differentiation are probably related to natural geographic barriers among populations, gene drift, and limited gene flow caused by restricted pollen flow and seed flow. A Mantel test indicated that geographic distances were significantly correlated with genetic distances. The UPGMA phenogram based on Nei’s unbiased genetic distances and the result of three-dimensional model plots performed by principal coordinate analysis also supported the correlation. Altitude, however, did not have any clear effect on genetic differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sea buckthorn, genus Hippophae L., is a dioecious, wind-pollinated pioneer plant with significant economic value. It is an ideal candidate for soil and water conservation and also plays an important role in agriculture, nutrition, medicine, and ornaments (Eliseev et al. 1989; Yao and Tigerstedt 1994; Singh et al. 1997). This important resource plant is attracting more and more attention in China, and its uncontrolled use has already led to deforestation and loss of genetic diversity. For the sake of protecting the genetic diversity of the sea buckthorn and its restoration, it is urgent to understand the genetic differentiation of this natural taxon. During recent decades, many studies have been undertaken on sea buckthorn, concentrating mainly on its origin, evolution, and phylogenetic relationships (Bartish et al. 2000, 2006; Sun et al. 2002; Sheng et al. 2006). Although studies have examined genetic diversity and genetic differentiation in some Hippophae species (Yao and Tigerstedt 1993; Bartish et al. 1999; Sun et al. 2006), knowledge is still too limited to grasp the genetic diversity and population genetic structure of the entire genus.
During the evolutionary process, taxa in Hippophae spread along an eastern route and a western route. Two subspecies, H. rhamnoides ssp. yunnanensis and H. rhamnoides ssp. sinensis, dispersed along the eastern route. The ssp. yunnanensis, endemic to the Qinghai-Tibet plateau, grows on the edge of the southeastern distribution area of Hippophae in small areas of the Hengduan Mountains at altitudes of 2200–4000 m. The distribution of ssp. sinensis overlaps with ssp. yunnanensis toward the northern edge of the ssp. yunnanensis distribution range. We have detected high genetic diversity and low population genetic differentiation in ssp. sinensis (Sun et al. 2006). It would be interesting to compare the genetic differentiation pattern of the other subspecies. Tian et al. (2004) studied the population genetic structure of ssp. yunnanensis, but populations from one of the main distribution areas in Sichuan province were absent. To compare genetic differentiation in the two subspecies, this study sampled six populations from three provinces (Tibet, Yunnan, and Sichuan) and covered the whole distribution area of ssp. yunnanensis. The results will further our understanding of the origin of this subspecies and of the processes of geographic distribution, the origin, and the evolution of all the Hippophae taxa.
The RAPD technique is widely used for estimating genetic diversity and relationships in plant populations (Huff et al. 1993; Heum et al. 1994; Ge et al. 1999). In the case of sea buckthorn, it is also useful for clarification of taxonomy, sex determination, and population genetic structure (Persson and Nybom 1998; Bartish et al. 1999, 2000, 2002; Sheng et al. 2006).
In this study, RAPD analysis was used to investigate the genetic diversity and structure of six natural populations of H. rhamnoides ssp. yunnanensis. Our purpose is to determine the pattern of genetic differentiation of the subspecies, to detect the relationship between genetic distance and geographic distance, and to compare its levels of genetic differentiation with those of H. rhamnoides ssp. sinensis (Sun et al. 2006) and H. rhamnoides ssp. rhamnoides (Bartish et al. 1999). The results would contribute to a better understanding of the population genetics and the origin of this subspecies, as well as a guide for strategies for ssp. yunnanensis conservation, selection, and breeding.
Materials and Methods
Population Sampling
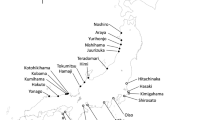
Hippophae rhamnoides ssp. yunnanensis is distributed in Tibet, Yunnan, and Sichuan provinces on the southeastern edge of the distribution area of the genus, growing mainly in Tibet, Yunnan, and Sichuan provinces, at altitudes of 2200–4000 m (Lian et al. 2000). Six natural populations covering the entire distribution of the subspecies were sampled on the southeastern edge of the Qinghai-Tibet plateau (Fig. 1).
From each population, 16–20 individuals were sampled randomly, including both sexes, for a total of 113 individuals (Table 1). Fresh leaves were harvested and dried with silica gel in the field.
DNA Extraction and PCR Amplification
DNA extraction followed Ge et al. (1999). PCR amplification used the same system as Qian et al. (2001). DNA amplification was performed in a Rapidcycler 1818 or 1605 (Idaho Tech) to select primers. Twelve arbitrary RAPD primers that could amplify reproducible and clear DNA bands were selected from 136 primers (obtained from Sangon Co.). DNA amplification was then performed in a Rapidcycler 1818. Amplification products were resolved by electrophoresis on 1.5% agarose gel stained with ethidium bromide in 0.5× TBE buffer and were imaged on Bio-Rad imaging devices (Gel Doc 2000 Gel Documentation System) supported by Quantity One (version 4.2). Molecular weights were estimated using a 100–3000 bp DNA ladder.
RAPD Analysis
Amplified DNA fragments were scored as 1 (present) or 0 (absent) for each DNA sample, forming a matrix of the RAPD phenotypes. Bands of identical size, amplified with the same primer, were considered to be homologous. The matrix was used in Popgene 32 (Yeh et al. 1997) to calculate the following genetic parameters: percentage of polymorphic bands (PPB), Nei’s gene diversity (h), Shannon’s index (I), population gene diversity (Ht), subpopulation gene diversity (Hs), and subpopulation differentiation (Gst). AMOVA was also used to calculate genetic variance components and their significance levels among populations and within populations.
Nei’s unbiased genetic distance (Nei 1978) was calculated among populations using Popgene. A matrix of Nei’s genetic distance was used to cluster the populations by the unweighted pair group method with arithmetic averaging (UPGMA) using Sahn in NTsys. A principal coordinate analysis was performed to get three-dimensional model plots, which provided an additional representation of genetic relationships among populations.
Mantel tests were computed to investigate the possible association between genetic and geographic distances, and between genetic distance and altitude.
Results
Genetic Diversity
The size of the amplified fragments ranged from 240 to 1800 bp, mostly between 300 and 1300 bp. The number of polymorphic loci amplified by each primer was distinctly high (Table 2). In particular, bands amplified by primers 276, 317, 352, 368, and 414 were all polymorphic. This indicated that ssp. yunnanensis showed high genetic diversity in this respect.
Of the 112 bands generated by the 12 primers, 104 were polymorphic, for a PPB at the subspecies level of 92.86%. Nei’s gene diversity (h = 0.255) and Shannon’s index (I = 0.397) also indicated a high level of gene diversity for the subspecies (Table 3).
Within-population gene diversity was relatively low (Table 3). The three parameters (PPB, h, and I) showed that genetic diversity in population Y2 was lower than in other populations. The population sampled in Tibet (Y1) maintained the highest genetic diversity within the population.
Genetic Differentiation Among Populations
The Gst value showed that 45.9% genetic variation resided among populations, indicating distinctly high among-population genetic divergence. Partitioning of molecular variance was analyzed using AMOVA (Table 4), which showed that 47.02% of the total variance existed among populations and 52.98% within populations. The genetic divergence among populations was statistically significant (P < 0.001).
Genetic Distance and Cluster Analysis
In the UPGMA phenogram based on Nei’s unbiased genetic distance matrix (Fig. 2), the geographically close individuals of populations Y3 and Y4 clustered together. The genetic distance between Y1 and other populations was comparatively great. The three-dimensional models plotted by principal coordinate analysis provided an additional representation of the genetic relationships among populations (Fig. 3). Except for individuals of Y3 and Y4, the four remaining populations were clearly separated in the first three dimensions.
Correlation Between Population Genetic Distance and Geographic Distance
To investigate a possible correlation between genetic distance and geographic distance, we compared Nei’s unbiased genetic distance matrix with a corresponding geographic distance matrix. The Mantel test showed that the two matrices were significantly correlated: r = 0.84, P = 0.977 (Prob. random Z < obs. Z, Z = normalized Mantel statistic). No significant correlation was found between genetic distance and altitude: r = −0.117, P = 0.324.
Discussion
Genetic Diversity
Li and Midmore (1999) reported that when the variation between genotypes is high, the use of a few primers will be sufficient to detect genetic differentiation. High genetic differentiation of the narrowly endemic Sinocalycanthus chinensis was revealed by RAPD analysis using 12 primers (Li and Jin 2006). In a study of 38 sesame populations with 7 primers, Ercan et al. (2004) obtained 61 bands for all populations, 78% of which were polymorphic. In this study, on the genetic variation of H. rhamnoides ssp. yunnanensis, 12 primers were used and 112 bands were generated, including 104 polymorphic bands, which was enough to allow for complete differentiation.
Bartish et al. (1999) concluded that the breeding system appears to be critical for explaining variation in within-population genetic diversity. As an outcrossing, wind-pollinated, woody species, H. rhamnoides tends to harbor comparatively high variability. Using RAPD analysis, Bartish et al. (1999) detected 89.7% polymorphic loci and 0.192 gene diversity (h) in 10 natural populations of H. rhamnoides ssp. rhamnoides. Sun et al. (2006) found that H. rhamnoides ssp. sinensis exhibited 88.78% polymorphic bands and Nei’s gene diversity of 0.204. A similar high level of genetic diversity is found here for ssp. yunnanensis (PPB = 92.86%, h = 0.255, I = 0.397).
As pointed out by Hamrick and Godt (1990), endemic species or species with restricted distribution usually tend to have relatively lower levels of variability within populations. There are other reports, however, that endemic species (even endangered species) might possess high levels of gene diversity (Cosner and Crawford 1994; Richter et al. 1994; Ge and Hong 1999; Liu et al. 2001). Hippophae rhamnoides ssp. yunnanensis, endemic to the Qinghai-Tibet plateau, is more narrowly distributed on the borders between Tibet, Sichuan, and Yunnan than ssp. rhamnoides and ssp. sinensis. It was expected that low variation would be observed, but we have detected high levels of genetic variation in ssp. yunnanensis. Surviving in a harsh environment, a species has to change in some aspects and accumulates more genetic variation in order to adapt itself to various ambient pressures (Li and Midmore 1999). The Qinghai-Tibet plateau is an area with high radiation, high altitude (above 3000 m on average), low pressure, and severe weather. These extreme environmental factors affect not only the morphological characters but also the genetics of ssp. yunnanensis. Consequently, in order to adapt itself to the environment of the Qinghai-Tibet plateau, ssp. yunnanensis accumulated high genetic divergence in its evolutionary history. The area where ssp. yunnanensis grows is mountainous with ravines, and species composition there is complex. Such surroundings form distinct spatial heterogeneity, and the resulting environmental selective pressures cause ssp. yunnanensis to vary greatly, with a high level of gene diversity.
It is noteworthy that the population genetic divergence of ssp. yunnanensis was slightly lower than that of ssp. sinensis and ssp. rhamnoides. For mainland and island populations of ssp. rhamnoides, the average gene diversity is 0.196 and 0.186, respectively (Bartish et al. 1999). The average PPB in ssp. sinensis was 55.81%, and the gene diversity (h) varied from 0.125 to 0.223, with an average of 0.168 (Sun et al. 2006). The genetic divergence parameters in this analysis were PPB = 46.28%, h = 0.139, and I = 0.214. For ssp. yunnanensis, distinct spatial heterogeneity caused by complex species composition, mountains, and ravines favored the accumulation of genetic divergence, although the microenvironment is homogeneous for individuals in a single population. In these surroundings, the individuals endure similar selective pressure and tend to evolve in similar directions. Ultimately, population genetic identity is comparatively high and genetic divergence is relatively low at the population level. In addition, sea buckthorn has a high capacity for vegetative reproduction through root suckers. Each individual sea buckthorn can produce 10 to several hundred new plants through root suckers (Lian et al. 2000). This high capacity for clonal reproduction may also be responsible for the relatively lower genetic diversity within populations of sea buckthorn species.
Some researchers have compared RAPD and ISSR methods and found that ISSR markers always reveal higher levels of polymorphism or reproducibility (Fang and Roose 1997; Parsons et al. 1997; Esselman et al. 1999; Qian et al. 2001; Wu et al. 2005). Using ISSR markers, Tian et al. (2004) detected that Nei’s unbiased genetic diversity in seven populations of ssp. yunnanensis averaged 0.1994, which was slightly higher than our result (0.139) using RAPD markers.
Young et al. (2002) found that the number of insects decreased and plant florescence shortened as altitude increased. Correspondingly, the efficiency of sexual reproduction was reduced and population genetic diversity tended to become lower. Some studies have suggested that there is no relationship between altitude and genetic diversity (Zhao et al. 2006; Li and Jin 2006). In this study, both Nei’s gene diversity and Shannon’s index indicated that the relatively high-altitude populations of Y2 (3800 m) and Y6 (3830 m) showed the lowest gene diversity (h = 0.111, I = 0.171 for Y2; h = 0.128, I = 0.203 for Y6), but no significant correlation between genetic diversity within populations and altitude was observed for all six populations. Genetic diversity data at altitudes of 3300–3800 m are not recorded in this paper; therefore, further studies are required on the relationship between population genetic diversity and altitude of ssp. yunnanensis.
High Genetic Differentiation
Bussell (1999) summarized the RAPD data of 35 species and found that, on average, 19.3% of the total genetic diversity resided among populations for 29 outbreeding species, and 62.5% of the total diversity resided among populations for six inbreeding species. As outcrossing species, the majority of the diversity was found within populations in both Hippophae rhamnoides ssp. sinensis (Gst = 18.3%; Sun et al. 2006) and ssp. rhamnoides (15.1% genetic variance existed among populations using AMOVA; Bartish et al. 1999). For ssp. yunnanensis, however, among-population genetic variation was considerably higher (Gst = 45.9%). Partitioning of molecular variance (AMOVA) showed similar results, with 47.02% of the total variance attributable to among-population divergence and 52.98% to divergence within populations (Table 4). Tian et al. (2004) also detected relatively high genetic differentiation of ssp. yunnanensis (Gst = 27.9%), which was a little lower than ours. Of the seven populations sampled by Tian et al., two were sampled in Tibet and the other five in a narrow area in Yunnan province with a small range of altitude (3270–3408 m). The short geographic distances and small difference in altitude favor gene flow among populations, which may result in relatively lower genetic differentiation among populations than in this study. Gene flow among populations has a significant influence on the distribution of genetic variation (Hamrick and Godt 1990). Species with limited gene flow usually show higher genetic variation among populations than those with broad gene flow. For wind-pollinated Hippophae rhamnoides ssp. yunnanensis, pollen flow and seed flow are two main factors in gene flow. Although the breeding system may broaden the gene flow over short distances, a natural geographic barrier composed of mountains and ravines, along with a complex species composition, restricts pollen flow and seed flow among populations of ssp. yunnanensis over long distances. As a result, among-population gene flow is limited. The isolated populations evolve independently in their local environment due to natural selection and eventually generate high genetic variation among populations. In addition, when gene flow is less than 1 (Nm < 1), genetic drift could lead to genetic differentiation (Wright 1931). The Nm value of ssp. yunnanensis was only 0.589 in this study. Therefore, the considerably higher genetic differentiation among populations in ssp. yunnanensis may be attributed to isolation between populations, limited gene flow, and genetic drift.
Hamrick et al. (1991) suggested that as over 50% of the total genetic variation resided among populations, six strategically placed populations should maintain 95% of their genetic diversity. In Hippophae rhamnoides ssp. yunnanensis, about half of the total genetic variation was observed among populations, implying that different populations across the distribution range of the species should be sampled to maintain high genetic diversity when a conservation strategy is implemented. In addition, as many individuals as possible should be collected in each population.
Correlation Between Population Genetic Distance and Geographic Distance
Some studies report no significant association between population genetic distance and geographic distance (Li et al. 1995; Wang 2003). There are exceptions though, in many plant species, significant correlations between geographic and genetic distances among populations have been found (Alpert et al. 1993; Gabrielsen et al. 1997; Graham et al. 1997; Tollefsrud et al. 1998). The isolation-by-distance hypothesis can explain these correlations (e.g., Dawson et al. 1995; Raybould et al. 1996; Comes and Abbott 2000). The Mantel test (r = 0.84, P = 0.977) in this paper indicated significant correlation between geographic distance and genetic distance for Hippophae rhamnoide ssp. yunnanensis. This can also be seen in the UPGMA phenogram based on Nei’s unbiased genetic distances. Geographically close populations cluster first. For instance, Y3 and Y4, sampled at Zhongdian, form a clade first (Fig. 2). Y1, which is geographically distant from the others, occupies a relatively isolated position. The results of the three-dimensional models plotted by principal coordinate analysis (Fig. 3) correspond with the UPGMA phenogram. Individuals of Y3 and Y4 could not be identified in the first three dimensions. The genetic distance between populations also provides an additional representation. On the contrary, no correlation was found between the genetic and geographic distances among populations in ssp. rhamnoides (Bartish et al. 1999) and ssp. sinensis (Sun et al. 2006).
Chen et al. (2008) detected a significantly positive correlation between genetic distance and altitude when examining the five populations and female subpopulations of H. rhamnoides in the Wolong Nature Reserve. According to Chen et al. (2008), altitudinal gradients may be the prime cause affecting the pattern of genetic variation. A Mantel test was also used between population genetic distances and altitudes in ssp. yunnanensis, but our results did not indicate that altitude has any clear effect on genetic differentiation (r = −0.117, P = 0.324).
Further studies of the reproductive biology, ecology, and population genetics utilizing other molecular techniques are currently under way and should yield valuable information for the conservation and utilization of this important resource species.
References
Alpert P, Lumaret RD, Giusto F (1993) Population structure inferred from allozyme analysis in the clone herb Fragaria chiloensis (Rosaceae). Am J Bot 80:1002–1006
Bartish IV, Jeppsson N, Nybom H (1999) Population genetic structure in the dioecious pioneer plant species Hippophae rhamnoides investigated by RAPD markers. Mol Ecol 8:791–802
Bartish IV, Jeppsson N, Bartish GI, Lu R, Nybom H (2000) Inter- and intraspecific genetic variation in Hippophae (Elaeagnaceae) investigated by RAPD markers. Plant Syst Evol 225:85–101
Bartish IV, Jeppsson N, Nybom H, Swenson U (2002) Phylogeny of Hippophae (Elaegnaceae) inferred from parsimony analysis of chloroplast DNA and Morphology. Syst Bot 27:41–54
Bartish IV, Kadereit JW, Comes HP (2006) Late Quaternary history of Hippophae rhamnoides L. (Elaeagnaceae) inferred from chalcone synthase intron (Chsi) sequences and chloroplast DNA variation. Mol Ecol 15(13):4065–4083
Bussell JD (1999) The distribution of random amplified polymorphic DNA (RAPD) diversity amongst populations of Isotoma petraea (Lobeliaceae). Mol Ecol 8:775–789
Chen G, Wang Y, Zhao C, Korpelainen H, Li C (2008) Genetic diversity of Hippophae rhamnoides populations at varying altitudes in the Wolong Natural Reserve of China as revealed by ISSR markers. Silvae Genet 57(1):27–36
Comes HP, Abbott RJ (2000) Random amplified polymorphic DNA (RAPD) and quantitative trait analyses across a major phylogeographic break in the Mediterranean ragwort Senecio gallicus Vill. (Asteraceae). Mol Ecol 9:61–76
Cosner ME, Crawford DL (1994) Comparisons of isozyme diversity in three rare species of Coreopsis (Asteraceae). Syst Bot 19(3):350–358
Dawson IK, Simon AJ, Waugh R, Powell W (1995) Diversity and genetic differentiation among subpopulations of Gliricidia sepium revealed by PCR-based assays. Heredity 74:10–18
Eliseev IP, Mazaeva EY, Malena TV, Ivashin II (1989) Formovae raznoobrazie nekotorykh populyatsii Hippophae rhamnoides L. v Kirgizskoi SSR. Rast Resursy 20:502–509
Ercan AG, Taskin M, Turgut K (2004) Analysis of genetic diversity in Turkish sesame (Sesamum indicum L.) populations using RAPD markers. Genet Resour Crop Evol 51:599–607
Esselman EJ, Li JQ, Crawford D, Winduss JL, Wolfe AD (1999) Clonal diversity in the rare Calamagrostis porteri ssp. insperata (Poaceae): comparative results for allozymes and random amplified polymorphic DNA (RAPD) and inter-simple sequence repeat (ISSR) markers. Mol Ecol 8:443–451
Fang DQ, Roose ML (1997) Identification of closely related Citrus cultivars with inter-simple sequence repeat markers. Theor Appl Genet 95:408–417
Gabrielsen TM, Bachmann K, Jakobsen KS, Brochmann C (1997) Glacial survival does not matter: RAPD phylogeography of Nordic Saxifraga oppositifolia. Mol Ecol 6:831–842
Ge S, Hong DY (1999) Studies of morphological and allozyme variation of the endangered Adenophora lobophylla and its widespread congener A. potaninii. Acta Genet Sin 26(4):140–147 (in Chinese)
Ge S, Oliveira GCX, Schaal BA, Gao LZ, Hong DY (1999) RAPD variation within and between natural populations of the wild rice Oryza rufipogon from China and Brazil. Heredity 82:638–644
Graham J, Squire GR, Marshall B, Harrison RE (1997) Spatially dependent genetic diversity within and between colonies of wild raspberry Rubus idaeus detected using RAPD markers. Mol Ecol 6:1001–1008
Hamrick JL, Godt MJW (1990) Allozyme diversity in plant species. In: Brown AHD et al (eds) Plant population genetics, breeding and genetic resources. Sinauer Associates, Inc., Sunderland, pp 43–63
Hamrick JL, Godt MJW, Murawski DA, Loveless MD (1991) Correlations between species and allozyme diversity: implications for conservation biology. In: Falk DA, Holsinger KE (eds) Genetics and conservation of rare plants. Oxford University Press, New York, pp 75–86
Heum M, Murrhy JP, Phillips TD (1994) A comparison of RAPD and isozyme analyses for determining the genetic relationships among Avena sterilis L. accessions. Theor Appl Genet 87:689–696
Huff DR, Peakall R, Smouse PE (1993) RAPD variation within and among natural populations of outcrossing buffalograss [Buchloë dactyloedes (Nutt.) Engelm.]. Theor Appl Genet 86:927–934
Li JM, Jin ZX (2006) High genetic differentiation revealed by RAPD analysis of narrowly endemic Sinocalycanthus chinensis Cheng et S.Y. Chang, an endangered species of China. Biochem Syst Ecol 34:725–735
Li M, Midmore DJ (1999) Estimating the genetic relationships of Chinese water chestnut (Eleocharis dulcis (Burm. f.) Hensch) cultivated in Australia, using Random amplified polymorphic DNAs (RAPDs). J Hort Sci Biotech 74:224–231
Li J, Tao Y, Zheng SZ, Zhou JL (1995) Isozymatic differentiation in local population of Glycine soja Sieb. & Zucc. Acta Bot Sin 37:669–676
Lian YS, Lu SG, Xue SK, Chen XL (2000) Biology and chemistry of the genus Hippophae. Gansu Science & Technology Press, Lanzhou, pp 1–226 (in Chinese)
Liu ZL, Li S, Yan GQ, Song Y, Zhao GF (2001) Genetic structure and intraspecific genetic polymorphisms in natural populations of Psathyrostachys huashanica. Acta Genet Sin 28(8):769–777
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Parsons BJ, Newbury HJ, Jackson MT, Ford-Lloyd BV (1997) Contrasting genetic diversity relationships are revealed in rice (Oryza sativa L.) using different marker types. Mol Breed 3:115–125
Persson HA, Nybom H (1998) Genetic sex determination and RAPD marker segregation in the dioecious species sea buckthorn (Hippophae rhamnoides L.). Hereditas 129:45–51
Qian W, Ge S, Hong DY (2001) Genetic variation within and among populations of a wild rice Oryza granulata from China detected by RAPD and ISSR markers. Theor Appl Genet 102:440–449
Raybould AF, Mogg RJ, Clarke RT (1996) The genetic structure of Beta vulgaris ssp. inaritima (sea beet) populations: RFLPs and isozymes show different pattern of gene flow. Heredity 77:245–250
Richter TS, Soltis PS, Soltis DE (1994) Genetic variation within and among populations of the narrow endemic Delphinium viridescens (Ranunculaceae). Am J Bot 81(8):1070–1076
Sheng HM, An LZ, Chen T, Xu SJ, Liu GX, Zheng XL, Pu LL, Liu YJ, Lian YS (2006) Analysis of the genetic diversity and relationships among and within species of Hippophae (Elaeagnaceae) based on RAPD markers. Plant Syst Evol 260:25–37
Singh V, Singh B, Awasthi CP (1997) Studies on distribution, taxonomy and nutritional values of sea buckthorn growing in dry temperate Himalayas. In: Lu S et al, (eds) Worldwide research & development of sea buckthorn. China Science & Technology Press, Beijing, pp 52–59
Sun K, Chen XL, Ma RJ, Li CB, Wang Q, Ge S (2002) Molecular phylogenetics of Hippophae L. (Elaeagnaceae) based on the internal transcribed spacer (ITS) sequences of nrDNA. Plant Syst Evol 235:121–134
Sun K, Chen W, Ma RJ, Chen XL, Li A, Ge S (2006) Genetic variation in Hippophae rhamnoides ssp. sinensis (Elaeagnaceae) revealed by RAPD markers. Biochem Genet 44(5/6):186–197
Tian CJ, Nan P, Shi SH, Chen JK, Zhong Y (2004) Molecular genetic variation in Chinese populations of three subspecies of Hippophae rhamnoides. Biochem Genet 42:259–267
Tollefsrud MM, Bachmann K, Jakobsen KS, Brochmann C (1998) Glacial survival does not matter—II: RAPD phylogeography of Nordic Saxifraga cespitosa. Mol Ecol 7:1219–1232
Wang Q (2003) Genetic diversity of three species of Ligularia (Asteraceae) in eastern of Qinghai-Tibet plateau of China. M.D. Thesis, pp 14–19 (in Chinese)
Wright S (1931) Evolution in Mendelian population. Genetics 16:91–159
Wu W, Zheng YL, Chen L, Wei YM, Yang RW, Yan ZH (2005) Evaluation of genetic relationships in the genus Houttuynia Thunb. in China based on RAPD and ISSR markers. Biochem Syst Ecol 33:1141–1157
Yao Y, Tigerstedt PMA (1993) Isozyme studies of genetic diversity and evolution in Hippophae. Genet Resour Crop Evol 40:153–164
Yao Y, Tigerstedt PMA (1994) Genetic diversity in Hippophae and its use in plant breeding. Euphytica 77:165–169
Yeh EC, Yang RC, Boyle TBJ, Ye ZH, Mao JX (1997) Popgene, the user-friendly shareware for population genetic analysis. Edmonton: molecular biology and biotechnology centre. University of Alberta, Canada
Young AG, Hill JH, Murray BG, Peakall R (2002) Breeding system, genetic diversity and clonal structure in the subalpine forb Rutidosis leiolepis F. Muell. (Asteraceae). Biol Conserv 106:71–78
Zhao QF, Li QX, Ma SR, Cui Y, Wang G (2006) RAPD analysis of genetic diversity of Kobresia humilis along the eastern of Qinghai-Tibet plateau of China. Acta Ecol Sin 26:2494–2501 (in Chinese)
Acknowledgments
Prof. Xin-Ping Wang (www.amaurobiidae.com) at the University of Florida provided comments on the manuscript. This research was supported by the National Natural Science Foundation of China (30960029), Science and Knowledge Innovation Project of Northwest Normal University (NWNU-KJCXGC-03-49).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, W., Su, X., Zhang, H. et al. High Genetic Differentiation of Hippophae rhamnoides ssp. yunnanensis (Elaeagnaceae), a Plant Endemic to the Qinghai-Tibet Plateau. Biochem Genet 48, 565–576 (2010). https://doi.org/10.1007/s10528-010-9339-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-010-9339-y