Abstract
Egeria densa Planchon (Hydrocharitaceae) is a submerged macrophyte native to South America. It forms part of a new suite of invasive aquatic plants that has benefited from open nutrient-rich freshwater systems following the successful biological control of floating aquatic plants in South Africa. The specificity of the leaf-mining fly, Hydrellia egeriae Rodrigues (Diptera: Ephydridae) was tested, using traditional laboratory host-specificity testing (i.e., no-choice and paired choice). Only one non-target species, Lagarosiphon major Deeming (Hydrocharitaceae) supported larval development during pair-choice tests. In order to avoid the rejection of a safe and potentially effective agent, continuation (i.e., multiple generations) tests were conducted to measure the ability of the non-target species to nutritionally support a population indefinitely. None of these species could sustain a viable agent population for more than three generations. Laboratory host-specificity tests are limited as they exempt certain insect-host behaviours. To enhance the interpretation of host-specificity results, a risk assessment was conducted using agent preference (i.e., choice tests) and performance (i.e., choice and continuation tests) results. The feeding and reproductive risk that H. egeriae poses to non-target species is below 2%. Based on these findings, permission for its release in South Africa has been obtained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aquatic weed Egeria densa Planchon (Hydrocharitaceae) is a freshwater plant, native to Brazil and temperate and subtropical areas of Argentina and Uruguay (Cook and Urmi-König 1984). Egeria densa is considered a vigorous and highly invasive plant of freshwater ecosystems outside its native range, rapidly producing dense infestations and swiftly colonising previously unaffected areas (Yarrow et al. 2009; Cabrera Walsh et al. 2013; Cook and Urmi-König 1984). The successful control of aquatic invasive weeds can be difficult to achieve using traditional methods such as mechanical and chemical control, which are often only effective in the short term. The physical removal of E. densa from waterways using water-level drawdowns or machinery can be counter-productive, facilitating the dispersal of the weed through fragmentation (Gettys et al. 2014; Hussner et al. 2017). In addition, the use of herbicide control in freshwater systems is increasingly deemed unsuitable due to its negative environmental effects on non-target species (Coetzee and Hill 2012).
During a national review of invasive aquatic weeds in South Africa (Coetzee et al. 2011), E. densa was identified for biocontrol as part of a rapid response to its range expansion. Hydrellia egeriae Rodrigues (Diptera: Ephydridae) has been identified as a promising agent due to its wide distribution in the native range, as well as significant oviposition and feeding on E. densa. Native range host specificity tests were conducted to establish the potential safety of H. egeriae (Cabrera Walsh et al. 2013). The results revealed that H. egeriae showed a clear preference for E. densa. However, the fly also developed on two other species within the same family: Egeria naias Planchon, and Elodea callitrichoides Rich. Casp. Species from the genera Egeria and Elodea do not occur naturally in South Africa (Cabrera Walsh et al. 2013) and given the specificity and favourable developmental attributes of H. egeriae, the fly was imported into South Africa in September 2014 for quarantine host-specificity testing.
Host-specificity testing forms the foundation of any biocontrol program. Despite the high safety record of released weed biocontrol agents (Hinz et al. 2019), concern for non-target effects by regulatory authorities, the general public and some scientists have been a major driving force for extensive refinement of host-specificity methodology. Traditional laboratory host-specificity tests include starvation (no-choice), choice, multi-choice and choice minus target tests, and less frequently used continuation (i.e., multiple generation) tests and time dependent tests (Marohasy 1998; van Driesche and Murray 2004). Choice tests although somewhat limited are valuable, creating a rank order of preference of plants that should be considered hosts. In some cases, further testing is required to examine the suitability of a host to support a biocontrol agent population over the long-term. Continuation tests are not common practice in classical biocontrol and often extend for long periods of time. These tests measure the ability of the host plant to nutritionally support a population indefinitely (Buckingham and Okrah 1993; Coetzee et al. 2003; Day et al. 2016). For example, choice tests with the sap-sucking mirid, Eccritotarsus eichhorniae Henry (Hemiptera: Miridae), illustrated an oviposition preference for its host plant, Eichhornia crassipes (Martius) Solms-Laubach (Pontederiaceae) compared to its family member Pontederia cordata L. The number of progeny that developed on E. crassipes was 13 times higher than for P. cordata. However, nymphs did not show a clear preference for E. crassipes, and continuation tests indicated that P. cordata was suitable to maintain a viable population over five generations (Tipping et al. 2018). Continuation tests can also tease out some of the limitations of laboratory host-specificity testing (Marohasy 1998). Buckingham and Okrah (1993) used continuation tests to establish that the non-target species, Potamogeton crispus L. (Potamogetonaceae), was unable to sustain Hydrellia pakistanae Deonier (Diptera: Ephydridae), a biocontrol agent for Hydrilla verticillata (L.f.) Royle (Hydrocharitaceae), for more than eight generations. Following the agent’s release, there have been no records of fly damage to P. crispus in the field.
Spill-over may occur temporarily where biocontrol agents cause a crash in the target weed population, and continuation tests can give an indication of how long the biocontrol agent could survive on the non-target species. It is important to note that continuation tests may fail to identify impact to non-target species when both target weed and non-target species overlap geographically. Therefore, short-term spill-over events have been simulated in pre-release experiments before. When transferred to non-target species after being fed with its target weed, adult longevity and female fecundity of Bikasha collaris Baly. (Coleoptera: Chrysomelidae), a biocontrol agent for Chinese tallowtree (Triadica sebifera L.), was comparable to no-choice tests (Wheeler et al. 2017). Ultimately, all tests conducted should model the ecological context in which the agents will interact with the potential hosts (Louda et al. 2003; Briese 2005), and interpretation of results should be carefully considered to ensure they are representative of the natural host-range or field host specificity (Cullen 1990; Balciunas et al. 1996; Cruttwell McFadyen 2003, Marohasy 1998).
Extrapolating laboratory results (i.e., the fundamental host range of the agent) to its realised host range can be challenging. Factors such as small cage sizes, bypassing steps in host location and agent experience or learning may produce agent behaviour that would not occur under natural conditions (Sheppard et al. 2005). Native range host-specificity testing is useful in making such predictions, but can be limited as it may not always include test species of the target region (Briese 2005). Risk assessment can enhance field-predictions of a potential biocontrol agent (Paynter et al. 2015). It uses the agent’s host-specificity results on non-target species relative to the target weed to calculate risk scores. These scores represent the feeding and developmental risk that the agent poses to each non-target species in the field (Wan and Harris 1997). Because risk assessment scores are standardized and easier to interpret, they can also be used as a tool to better communicate laboratory results to regulatory authorities, stakeholders and the general public.
In this study, in addition to choice and no-choice tests, we also conducted continuation tests to determine if non-target species used during choice-tests are physiologically suitable to sustain agent populations in the field. We also used risk assessment to determine the risk of releasing H. egeriae. We present the results of host specificity tests on H. egeriae, together with a risk assessment pertaining to the release of H. egeriae in South Africa.
Materials and methods
Host plant culture
Plant material was collected throughout the years 2014 and 2015 from the Kouga River, Patensie, Eastern Cape, South Africa (S 33°44′ 54.622″; E 24°38′ 7.605″) and cultured in a flow-through system in a polytunnel at the Waainek CBC Facility in Makhanda, South Africa. Thirty shoots, 20 cm in length, were individually planted in 13.5 l round tubs (41 cm × 41 cm × 24 cm) with pond sediment and the slow release fertilizer Multicote™ (Haifa) at a ratio of 0.7 g per 1 kg sediment. A 1 cm silica sand layer was placed over the sediment to minimize water clouding and algal growth. Planted tubs were placed in 600 l tanks connected to a flow-system. Plants were given a fluid nutrient stock solution every third month that consisted of calcium chloride (91.7 mg l−1), magnesium sulphate (69.0 mg l−1), sodium bicarbonate (58.4 mg l−1) and potassium bicarbonate (15.4 mg l−1) (Smart and Barko 1985). Plant material from this E. densa culture was used for all of the experiments in this study.
Insect culture
In September 2014, H. egeriae was imported under permit (P0063110) from the Exotic and Invasive Weeds Research (EIW) facility of the Agricultural Research Service in California, USA to the Rhodes University Quarantine Facility in South Africa. The founder culture was initiated in May 2013 from one shipment that contained individuals from four different populations in Argentina (John Herr, pers. comm.; Guillermo Cabrera Walsh pers. comm.).
Biology of Hydrellia egeriae
Adults are between 1.3 and 3.0 mm in size, live on the water surface and feed on fungi, yeast, nectar and small and/or trapped insects. Females oviposit eggs on protruding E. densa leaves and have a lifespan of about 13 days (at 22 °C). Hydrellia egeriae immatures are fully aquatic and undergo three instars during which they mine on the photosynthetic tissue of E. densa leaves. Larvae mine on average 24.5 leaves. After 16 days of feeding, the third instar undergoes a non-feeding pre-pupa stage, before pupariation within an E. densa leaf. Adults emerge after ten days and float to the water surface in an air bubble.
In order to start a culture of the potential control agent in South Africa, H. egeriae larvae were placed in transparent boxes (41 cm × 17 cm × 29 cm) equipped with a mesh window and kept in a controlled environment of 22 ± 2 °C under fluorescent lighting (Osram Gro-Lux 58 W, 3700 lumens, 1.5 m) and a 12:12 L:D photoperiod. Each box was half-filled with spring water and contained 25 E. densa apical stems, 15 cm to 20 cm in length, and a floating Petri dish with a yeast hydrolysate/sugar mixture (4 g Bacto™ TC yeastolate per 7 g sugar per 10 ml H2O) (Buckingham and Okrah 1993). Immatures were left to complete development and newly emerged adults were collected with a mouth aspirator and transferred to new boxes to allow mating, oviposition and development. Every week, one new box was set up with newly emerged adults, during which boxes were checked for inconsistencies (e.g., fungal growths) to maintain a disease-free insect culture. Water and new plant material were added as needed. All tests conducted with H. egeriae were conducted in the Centre for Biological Control (CBC) quarantine facility and used individuals from this fly culture.
Host specificity
Test plants
Non-target plants for host-specificity testing were selected using the centrifugal phylogenetic method (Wapshere 1974) with modifications by Briese (2003). Phylogenetic trees of the order Alismatales (Petersen et al. 2015) and the family Hydrocharitaceae (Chen et al. 2012) were used to identify families and genera that are related to the target plant. Species of these families and genera that are present in South Africa were selected for testing (Table 1). One species, Myriophyllum spicatum L. (Haloragaceae), was selected on the basis of ecological similarity.
Prior to experimental set up, individual test plants were planted in 3 cm × 5 cm vials, containing sediment and a slow release fertilizer, MulticoteTM (Haifa) to a ratio of 0.7 g per 1 kg sediment. Plants were grown in 600 l tanks that are connected to a flow-system in a polytunnel at the Waainek CBC Facility, Makhanda. A fluid nutrient stock solution was added to the tanks to ensure healthy plant growth (Smart and Barko 1985). Rooted plants were used for host-specificity testing, and if not available, healthy leaves or plant fragments were used.
Three test species from the Hydrochariataceae, Lagarosiphon ilicifolius Obermeyer, Lagarosiphon verticillifolius Obermeyer and Ottelia exserta Ridley, could not be collected, despite extensive efforts. Lagarosiphon ilicifolius is from southern Africa (Mozambique, Namibia and Botswana) and exportation of these species into South Africa was problematic. Lagarosiphon verticillifolius and O. exserta could not be collected due to an extensive drought in 2015 and 2016 that resulted in low water levels in the restricted rivers and dams where they occur. These species are also geographically isolated and rarely found. Nonetheless, test species within the Hydrocharitaceae were well represented, including species from four genera that are more commonly found in South Africa.
Hydrellia egeriae individuals for testing
A combination of first instars (< 24 h old) and eggs were used for host-specificity tests. To obtain individuals, ten pairs of newly emerged adults were placed in a transparent box (41 cm × 17 cm × 29 cm), half-filled with 10 l spring water, 25 E. densa apical shoot tips and a yeast hydrolysate/sugar mixture (4 g Bacto™ TC yeastolate per 7 g sugar per 10 ml H2O) provided on a floating feeding station. Adults were allowed to mate and oviposit and leaves with eggs were harvested and placed in a Petri dish containing spring water. Five neonate larvae/eggs were transferred to test plants by excising the leaf material around it, and pinning the excised leaf with the larva/egg, onto the test plant. Eggs were checked for larval emergence after initiation of the replicate.
No-choice larval feeding
Test plants were individually placed in 600 ml containers (24 cm × 7.5 cm) filled with spring water. An excised E. densa leaf containing first instar/eggs was pinned to leaves on the test plants with minuten pins. Containers were enclosed with netting, held in place by an elastic band to prevent any H. egeriae adults from escaping. One replicate consisted of sufficient test plant material for feeding and development and five H. egeriae larvae/eggs. After 30 days, replicates were checked for larval mining and pupariation. Larval mining was determined by stereo microscope observation and recorded. The leaf area mined (¼, ½, ¾ or 1) as well as the total number of leaves for the test species were recorded in order to calculate the percentage of the test plant damaged. Survival was measured as the number of H. egeriae individuals that pupated on the test plant.
Paired choice larval feeding
Egeria densa and a test species were placed together in a 1.5 l container with spring water. Stems of the tests species were intertwined with each other. Excised E. densa leaves with first instars/eggs, were attached to a 1 cm × 1 cm piece of condensed sponge with a minuten pin and placed in the middle of the container to drift in the water over the test species. The sponge allowed for buoyancy while the instars/eggs were suspended just below the water surface, allowing them to choose their feeding site. The number of damaged leaves was recorded as well as the number puparia for each test plant.
Continuation test
Test species that supported agent development during paired choice tests were subjected to continuation tests. Thirty apical shoots of the test species were placed in a transparent culture container (41 cm × 17 cm × 29 cm) filled with spring water. To initiate the test, a total of 100 H. egeriae first instars/eggs on excised E. densa leaves were pinned to shoots of the test species and left to feed and develop. After 30 days, the container of each test species was checked for adult emergence every second day, during which the adults were removed with a mouth aspirator and placed into a new culture container containing the test species from which they emerged. Food (4 g Bacto™ TC yeastolate per 7 g sugar per 10 ml H2O) for adults was provided on a Petri dish. The continuation test for the target weed was conducted until F3, and for non-target species, until the population died out.
Risk assessment
Potential non-target effects (i.e., feeding and reproductive risk) posed by releasing H. egeriae were calculated using the agent’s feeding and survival result for each non-target species relative to the target weed, E. densa (Wan and Harris 1997). The following criteria were used: plant preference, plant acceptability, larval survival and number of F1 adults. The feeding risk for each non-target species was calculated as the product of the plant preference (i.e., mean percentage feeding on a non-target species relative to its host plant during choice tests) and plant acceptability (i.e., mean number of mined leaves during no-choice tests relative to its host plant). Similarly, the reproductive risk was calculated by multiplying the relative survival of H. egeriae on non-target species during no-choice tests to its host plant and the mean number of F1 adults that emerged from non-target species during continuation tests. Zero values were replaced with 0.001 to facilitate calculation of risk scores. Standard errors (± SE) for preference and performance scores were calculated using \(\sqrt {\frac{{p\left( {1 - p} \right)}}{n}}\), where p represents the risk score and n the total number of H. egeriae individuals used for the respective test plant during each host-specificity test.
Statistical analysis
All statistical analyses were conducted in the R environment version 3.2.3 (R Core Team 2014). The distribution of larval damage and survival for no-choice and choice feeding tests was tested for normality using the Shapiro–Wilk test. Due to the uneven distribution of all the dependent variables, a non-parametric Kruskal–Wallis test was used to determine statistical difference between test plants for larval feeding and survival during no-choice tests. The post-hoc Kruskal-Dunn test was used to identify significant differences (P < 0.05) between test plants during no-choice tests. A Wilcoxon rank sum test was used to determine statistical differences between plants during paired choice tests.
Results
No-choice larval feeding
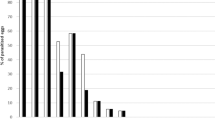
In total, 19 plant species in six families were tested. Hydrellia egeriae expressed significant preference for its host plant, E. densa. Larvae produced over three times more damage to E. densa leaves than any of the non-target species (Kruskal–Wallis test, \(\chi^{2}\)= 59.98; df = 5; P < 0.001) (Table 2). During the no-choice tests, H. egeriae mined only closely related species within the Hydrocharitaceae. These included L. major, L. muscoides, L. cordofanus, H. verticillata and V. spiralis. Egeria densa supported over five times more H. egeriae survival to adulthood compared to non-target species (Kruskal–Wallis test, \(\chi^{2}\)= 71.82; df = 5; P < 0.001) with a percentage of 82.22 ± 4.04% (Table 2). Non-target species that supported larval development were L. major, L. muscoides and V. spiralis with survival percentages of 12.00 ± 4.42%, 6.67 ± 5.12% and 3.53 ± 0.16%, respectively. Only species that supported agent survival during no-choice tests were subjected to choice larval feeding tests. Furthermore, 13 of the 19 non-target species tested under no-choice conditions incurred no larval mining and supported no agent development. Two of these species, Najas horrida and N. marina, are within the Hydrocharitaceae, the remainder belong to less closely related families that include the Potamogetonaceae, Alismataceae, Araceae, Aponogetonaceae and Haloragaceae (Table 1).
Paired choice larval feeding
During paired choice tests, H. egeriae preferred E. densa for feeding eight times more than the non-target species L. major (Wilcoxon rank sum test, W = 174; P < 0.001), L. muscoides (Wilcoxon rank sum test, W = 35; P = 0.002) and V. spiralis (Wilcoxon rank sum test, W = 16; P = 0.02) (Table 3). Larval survival followed the same trend with L. major (Wilcoxon rank sum test, W = 422.5; P < 0.001), L. muscoides (Wilcoxon rank sum test, W = 49; P < 0.001) and V. spiralis (Wilcoxon rank sum test, W = 25; P = 0.007) as significant inferior options for pupation. The percentage of H. egeriae that pupated in E. densa was over 13 times more than the non-target species L. major. Additionally, H. egeriae did not pupate in L. muscoides or V. spiralis.
Continuation test
The only test plant that could sustain a growing agent population was E. densa (Table 4). The mean number of H. egeriae instars that survived to F1 was 75.5 ± 4.5, which produced an F2 population of 217.5 ± 25.5 individuals. Lagarosiphon major was the only test plant that supported a viable population during the founder population, with 6.75 ± 3.9 adults developing unto adulthood. However, population growth was negative with no viable adults produced in the first generation.
Risk assessment
Despite some feeding and development on non-target species during no-choice, choice and continuation tests, risk assessment scores illustrated that the non-target risk posed by H. egeriae is very low. Relative to the target species, the feeding and reproductive risk of non-target species, L. major, is ten time less compared to E. densa (Table 5). Additionally, feeding and reproductive risk scores for L. muscoides and V. spiralis did not exceed 0.03%.
Discussion
Results from this quarantine-based study supports results from native range specificity testing, where H. egeriae expressed a clear preference for, and higher performance on its host plant during no-choice, choice and open field tests (Cabrera Walsh et al. 2013). Out of 19 non-target plant species tested, H. egeriae only mined five non-target species, all within the Hydrocharitaceae, and completed development on three of these non-target species. Under field conditions, starved larvae isolated from their host plant may cause temporary damage to L. major, L. muscoides, L. cordofanus, H. verticillata and V. spiralis. This may occur if H. egeriae disperses to new areas where the target weed is not available or where agent damage drastically reduced E. densa populations. As illustrated by the continuation tests, only one non-target species, L. major will be able to support a viable agent population. In a review on the efficiency of using relative performance scores to predict non-target effects, Paynter et al. (2015) found that non-target effects (e.g., spill-over, full utilization) were evident for risk scores above 0.20 (20%). Based on the risk assessment, L. major is the only non-target species on which H. egeriae poses a major feeding and reproductive risk with scores below 1.34%. In the field, H. egeriae would also have to compete with native Hydrellia species that feed on native Lagarosiphon species, for example, Hydrellia lagarosiphon Deeming (Diptera: Ephydridae), a widely distributed, host-specific, herbivore of L. major (Martin et al. 2013). Hybridization of biocontrol agents with related species has been recorded in four cases (Havill et al. 2012), and is an undesirable non-target effect. Yet, using an extensive systematic and ecological study of the genus Hydrellia, Deonier (1971) never encountered interbreeding of Hydrellia species, under either laboratory, or natural conditions. This suggests that hybridization of H. egeriae and H. lagarosiphon or any native Hydrellia species in the field is highly unlikely.
Specialist herbivores often use closely related species due to similar morphological and chemical traits (Futuyma and Agrawal 2009). A phylogenetic tree of the Hydrocharitaceae based on two plastid genes (rbcL and matK) and five mitochondrial genes (atp1, ccmB, cob, mttB and nad5) (Chen et al. 2012), indicates that the genera Lagarosiphon and Egeria are within the same clade, whereas Hydrilla and Vallisneria are located within a sister clade. The genus Lagarosiphon is from the Afrotropics. Species within the genus are morphologically similar to E. densa (Chen et al. 2012). The phylogenetic relatedness of the genus to E. densa predicted H. egeriae mining and development on L. major and L. muscoides during no-choice testing. Feeding and development on the further related V. spiralis support the hypothesis that no-choice tests can produce false-positives due to small cage sizes and interference with natural host finding behaviour (van Driesche and Murray 2004; Sheppard et al. 2005). In its native range, open field choice tests indicated that H. egeriae only colonized E. densa, and no leaf-mining or adults were recorded in V. spiralis (Cabrera Walsh et al. 2013).
Although the test plant list from this study is not phylogenetically complex, risk assessment scores have proven valuable in such cases. For example, biocontrol agents for the invasive weeds Solanum mauritianum Scopoli (Solanaceae) and Tithonia diversifolia (Hemsl.) A. Gray (Asteraceae) showed considerable preference and performance on non-target species during host-specificity testing, but had inferior feeding and reproductive risk scores compared to the target weed (Olckers 2000; Mphephu et al. 2017).
Concerted efforts should be made to fine tune testing methodology using the latest information and concepts, and drawing on past experiences to avoid repeating failures. No-choice and choice tests will continue to be the mainstay of laboratory host-specificity testing, have been used to adequately predict agent safety (Paynter et al. 2015) and further utilized in risk assessments (Olckers 2000; Mphephu et al. 2017). Although less frequently used, continuation tests add strength to host-specificity test results (Buckingham and Okrah 1993; Coetzee et al. 2003; Tipping et al. 2018), and as shown here, can be used in risk assessment to predict the reproductive risk of a biocontrol agent. Based on the findings from this study, permission for the release of H. egeriae in South Africa has been obtained.
References
Balciunas JK, Burrows DW, Purcell MF (1996) Comparison of the physiological and realized host-ranges of a biological control agent from Australia for the control of the aquatic weed, Hydrilla verticillata. Biol Control 7:148–158
Briese DT (2003) The centrifugal phylogenetic method for selection of test plants for host-specificity testing of biological control agents: Can it and should it be modernized? In: Jacob HS, Briese DT (eds) Improving the selection, testing, and evaluation of weed biological control agents. CRC for Australian Weed Management, Glen Osmond, pp 23–33
Briese DT (2005) Translating host-specificity test results into the real world: the need to harmonize the yin and yang of current testing procedure. Biol Control 35:208–214
Buckingham GR, Okrah EA (1993) Biological and host range studies with two species of Hydrellia (Diptera: Ephydridae) that feed on hydrilla, technical report A-93-7. U.S. Army Engineer Waterways Experiment Station, Vicksburg
Cabrera Walsh G, Ym Dalto, Mattioli FM, Carruthers RI, Anderson LW (2013) Biology and ecology of Brazilian elodea (Egeria densa) and its specific herbivore, Hydrellia sp., in Argentina. BioControl 58:133–147
Chen LY, Chen JM, Gituru RW, Wang QF (2012) Generic phylogeny, historical biogeography and character evolution of the cosmopolitan aquatic plant family Hydrocharitaceae. BMC Evol Biol 12(30):1–12
Coetzee JA, Hill MP (2012) The role of eutrophication in the biological control of water hyacinth, Eichhornia crassipes, in South Africa. BioControl 57:247–261
Coetzee JA, Byrne M, Hill MP (2003) Failure of Eccritotarsus catarinensis, a biological control agent of waterhyacinth, to persist on pickerelweed, a non-target host in South Africa after forced establishment. Biol Control 28:229–236
Coetzee JA, Bownes A, Martin GD (2011) Prospects for the biological control of submerged macrophytes in South Africa. Afr Entomol 19(2):469–487
Cook CDK, Urmi-König K (1984) A revision of the genus Egeria (Hydrocharitaceae). Aquat Bot 19:73–96
Cruttwell McFadyen RE (2003) Does ecology help in the selection of biocontrol agents? In: Jacob HS, Briese DT (eds) Improving the selection, testing and evaluation of weed biological control agents. CRC for Australian Weed Management, Glen Osmond, pp 5–9
Cullen JM (1990) Current problems in host-specificity screening. In: Delfosse ES (ed.) Proceedings of the VII international symposium on biological control of weeds, Instituto Sperimentale per la Patologia Vegetale (MAF), Rome, Italy, pp. 27–36
Day MD, Riding N, Senaratne KADW (2016) The host specificity and climate suitability of the gall fly Cecidochares connexa (Diptera: Tephritidae), a potential biological control agent for Chromolaena odorata (Asteraceae) in Australia. Biocontrol Sci Technol 26(5):691–706
Deonier DL (1971) A systematic and ecological study of Nearctic Hydrellia (Diptera: Ephydridae). Smithson Contrib Zool 68:1–147
Futuyma DJ, Agrawal AA (2009) Macroevolution and the biological diversity of plants and herbivores. Proc Natl Acad Sci 106(43):18054–18061
Gettys LA, Haller WT, Petty DG (2014) Biology and control of aquatic plants, 3rd edn. Aquatic Ecosystem Restoration Foundation, Marietta, p 238
Havill NP, Davis G, Mausel DL, Klein J, McDonald R, Jones C, Fischer M, Salom S, Caccone A (2012) Hybridization between a native and introduced predator of Adelgidae: an unintended result of classical biological control. Biol Control 63:359–369
Hinz HL, Winston RL, Schwarzländer M (2019) How safe is weed biological control? A global review of direct nontarget attack. Q Rev Biol 94(1):1–27
Hussner A, Stiers I, Verhofstad MJJM, Bakker ES, Grutters BMC, Haury J, van Valkenburg JLCH, Brundu G, Newman J, Clayton JS, Anderson LWJ, Hofstra D (2017) Management and control methods of invasive alien freshwater aquatic plants: a review. Aquat Bot 136:112–137
Louda SA, Pemberton RW, Johnson MT, Follett PA (2003) Non-target effects—the Achilles heel of biological control? Retrospective analysis to reduce risk associated with biocontrol introductions. Ann RevEntomol 48:365–396
Marohasy J (1998) The design and interpretation of host-specificity tests for weed biological control with particular reference to insect behavior. Biocontrol News Inf 19:13–20
Martin GD, Coetzee JA, Baars JR (2013) Hydrellia lagarosiphon Deeming (Diptera: Ephydridae), a potential biological control agent for the submerged aquatic weed, Lagarosiphon major (Ridley) Moss (Hydrocharitaceae). Afr Entomol 21(1):151–160
Mphephu TE, Olckers T, Simelane DO (2017) The tortoise beetle Physonota maculiventris (Chrysomelidae: Cassidinae) is suitable for release against weedy Mexican sunflower Tithonia diversifolia (Asteraceae) in South Africa. Biocontrol Sci Technol 27(4):510–524
Olckers T (2000) Biology, host specificity and risk assessment of Gargaphia decoris, the first agent to be released in South Africa for the biological control of the invasive tree Solanum mauritianum. Biocontrol 45:373–388
Paynter Q, Fowler SV, Gourley AH, Peterson PG, Smith LA, Winks CJ (2015) Relative performance on test and target plants in laboratory tests predicts the risk of non-target attack in the field for arthropod weed biocontrol agents. Biol Control 80:133–142
Petersen G, Seberg O, Cuenca A, Stevenson DW, Thadeo M, Davis JI, Grahams D, Ross TG (2015) Phylogeny of the Alismatales (Monocotyledons) and the relationship of Acorus (Acorales?). Cladistics 32(2):1–19
R Core Team (2014) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. Available via R Project: https://www.r-project.org
Sheppard AW, van Klinken RD, Heard TA (2005) Scientific advances in the analysis of direct risks of weed biological control agents to nontarget plants. Biol Control 35(3):215–226
Smart RM, Barko J (1985) Laboratory culture of submersed freshwater macrophytes on natural sediments. Aquat Bot 21:251–263
Tipping PW, Foley JR, Gettys LA, Minteer CA (2018) Assessing the risk of Eccritotarsus eichhorniae to pickerelweed, Pontederia cordata in North America. Biocontrol Sci Technol 28(4):299–308
van Driesche RG, Murray TJ (2004) Overview of testing schemes and designs used to estimate host ranges. In: van Driesche RG, Reardon R (eds) Assessing host ranges for parasitoids and predators used for classical biological control: a guide to best practice. USDA, Forest Health Technology Enterprise Team, Morgantown, pp 68–89
Wan FH, Harris P (1997) Use of risk analysis for screening weed biocontrol agents: Altica carduorum guer. (Coleoptera: Chrysomelidae) from China as a biocontrol agent of Cirsium arvense (L.) Scop. in North America. Biocontrol Sci Technol 7:299–308
Wapshere AJ (1974) A strategy for evaluating the safety of organisms for biological weed control. Annu Appl Biol 77:201–211
Wheeler GS, Duncan JG, Wright S (2017) Predicting spillover risk to non-target plants pre-release: Bikasha collaris a potential biological control agent of Chinese tallowtree (Triadica sebifera). Biol Control 108:16–21
Yarrow M, Marín VH, Finlayson M, Tironi A, Delgado LE, Fischer F (2009) The ecology of Egeria densa Planchon (Lipliopsida: Alismatales): a wetland ecosystem engineer? Rev Chil Hist Nat 82:299–313
Acknowledgements
This research was funded through the Department of Environmental Affairs, Natural Resource Management Programme’s Working for Water programme. Further funding for this work was provided by the South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation of South Africa. Thanks are extended to the editors and anonymous reviewers for their patient guidance and critical comments, which has improved the quality of this manuscript. Thanks are due to Dr Emily Strange, Dr Grant Martin and Dr Guillermo Cabrera Walsh for their assistance in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: S. Raghu
Rights and permissions
About this article
Cite this article
Smith, R., Mangan, R. & Coetzee, J.A. Risk assessment to interpret the physiological host range of Hydrellia egeriae, a biocontrol agent for Egeria densa. BioControl 64, 447–456 (2019). https://doi.org/10.1007/s10526-019-09942-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-019-09942-4