Abstract
Characterization of new Bacillus thuringiensis strains is a valuable tool to discover novel insecticidal toxins and to manage resistance problems. In this study, seven Iranian Bt strains were selected according to their toxicity against Plodia interpunctella, to be thoroughly characterized based on their toxicity, protein profiling, proteomic analysis, gene content and β-exotoxin production. The toxicity was assessed by insect bioassays and cell viability assays (a less cost, time and material consuming technique), using four lepidopteran pests and four lepidopteran cell lines from Trichoplusia ni (Hi5), Helicoverpa zea (HzGUT), Spodoptera exigua (UCR-SE) and Spodoptera frugiperda (Sf21). The selected Bt strains showed similar protein electrophoretic profiles, but differed in toxicity. LC–MS/MS analysis of solubilized crystal proteins and gene content analyses (PCR screening) were compared and correlated with the toxicity results. Based on our data, three Bt strains could be considered as candidates for development of future bioinsecticides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacillus thuringiensis (Bt), a gram positive, rod-shaped and spore forming bacterium has proven to be a useful entomopathogen for microbial control of pests. Nowadays, Bt-based insecticides or transgenic plants expressing Bt insecticidal proteins are extensively used worldwide as a powerful alternative to chemical treatments (Bravo et al. 2011).
Bt synthesizes parasporal crystal proteins that are toxic to many insects, nematodes, mites, protozoa and human cancer cells (Schnepf et al. 1998; van Frankenhuyzen 2009; Ohba et al. 2009). Due to the high specificity, safety and specific insecticidal activity of the Bt toxins, several studies have been focused on Bt isolation from diverse sources (Bel et al. 1997; Uribe et al. 2003; Berόn and Salerno 2006; Seifinejad et al. 2008; Djenane et al. 2017). But the extensive application of Bt-based products has raised some concerns about the appearance of insect resistance, which has been reported in some species (Ferré et al. 1995). Thus, characterization of new Bt strains is necessary to discover novel toxins with new specificities or with a broader range of insecticidal activity, and to better manage insect resistance problems (Ferré et al. 1995; Juárez-Pérez et al. 1997).
In many Bt screening research works, it has been shown that PCR-based characterization is helpful to assess gene content and therefore to predict the toxicity of the new Bt isolates (Juárez-Pérez et al. 1997; Ferrandis et al. 1999). However, this quick and accurate technique lacks the ability to indicate the expression level of the detected genes. Further, the relative proportion of each protein in parasporal crystals can play an important role in the insecticidal potency of the Bt strain. Therefore, liquid chromatography and tandem mass spectrometry (LC-MS/MS) analysis could be considered as a good method to detect protein composition of the newly analyzed Bt strains and to predict their toxicity. By considering the interaction between different toxins produced by a strain in insect midgut, bioassays provide complementary and necessary characterization information. Due to the money, time and material costs associated to insect rearing and time-consuming characteristics of insect bioassays, cell-based assays have been employed for toxicity characterization of Bt strains or toxins (Kwa et al. 1998; Gringorten et al. 1999; Willcoxon et al. 2016). Moreover, the use of insect cell lines allows researchers to work with insect species without considering regulatory restrictions or prohibitions.
The aim of this study was to characterize new Bt strains isolated from soil and lepidopteran infected larvae to assess their possible potential as biopesticides against lepidopteran pests. For this purpose, strains with diverse larvicidal activity against Indian meal moth, Plodia interpunctella (Hubner) (Lep.: Pyralidae), were characterized according to their protein profile and gene content (cry, cyt, vip and ps genes). Afterwards the cry gene expression was verified by LC–MS/MS analysis of solubilized crystal proteins. The spectrum of lepidopteran toxic activity was assessed against Spodoptera exigua (Hubner) (Lep.: Noctuidae), Mamestra brassicae (L.) (Lep.: Noctuidae), Grapholita molesta (Busck) (Lep.: Tortricidae) and Ostrinia nubilalis (Hubner) (Lep.: Crambidae) first instar larvae. Then, to complete the analysis of the insecticidal activity, in vitro toxicity assays with lepidopteran cell lines (cytocidal activity) were performed. As a final step in characterization, the production of β-exotoxin was investigated as a necessary requirement to propose, at least, a new Bt strain as a candidate for commercial use.
Materials and methods
Bacterial strains
Seven Iranian Bt strains from the Bt stock of Insect Biological Control laboratory at the University of Tehran, were used in the study. The selected Bt strains were isolated from soil and diseased and dead larvae. Phase contrast microscopy was used to confirm the presence of the parasporal crystals and to determine their shape. The B. thuringiensis HD1 strain HD1S2005 (Bt HD1) (kindly provided by Valent Bioscience, Inc., Libertyville, IL, USA) and the B. thuringiensis subsp. thuringiensis strain HD2 (Berliner) (Bt HD2) (obtained from the Bacillus Genetic Stock Center, BGSC, Ohio State University, Ohio, USA), were used as reference standard strains.
Insect rearing
Plodia interpunctella was obtained from the Insect Physiology laboratory, Department of Plant Protection, University of Tehran (Iran), and reared for at least five years in Insect Biological Control laboratory at the same University. S. exigua and M. brassicae were obtained from the laboratory of Dr. Primitivo Caballero (Universidad Pública de Navarra, Spain). Grapholita molesta, was obtained from Entomos AG (Switzerland). Ostrinia nubilalis was obtained from the INRA (France). These insect colonies have been reared for at least three years in the laboratory of the University of Valencia (Spain) on artificial diet (McGaughey and Beeman 1988; Hernández-Martinez et al. 2008; Gomis-Cebolla et al. 2017; Bel et al., 2011), and kept at 25 ± 1 °C with 70 ± 5% RH with a16:8 h L:D photoperiod.
Toxicity bioassays
The insecticidal activity of seven Bt strains was assessed against P. interpunctella second instar larvae and first instar larvae of S. exigua, G. molesta, O. nubilalis and M. brassicae. The susceptibility of P. interpunctella larvae towards selected Bt strains was investigated in a single discriminating dose of 108 spores ml−1 based on previous analyses (data not shown) by food incorporation method. The assays with P. interpunctella were carried out in triplicate using 20 second instar larvae in each replicate and the mortality was recorded 72 h after treatment.
The susceptibility of S. exigua, G. molesta, O. nubilalis and M. brassicae first instar larvae to solubilized protoxins or to activated toxins (see next paragraph for solubilization and activation of the Cry proteins) at a single concentration of 1000 ng cm−2, was assessed by surface contamination method (Hernández-Martinez et al. 2008). The bioassays were repeated three times (48 larvae in each replicate). The buffer used for Bt crystal proteins solubilization (carbonate buffer 50 mM, 0.1 M NaCl, 10 mM dithiothreitol, pH 10.5) was used for negative controls. In order to compare the toxicity of the tested strains with previous studies, we selected Bt HD1 as reference strain. Bioassays were conducted at 25 ± 1 °C, 70 ± 5% RH and a16:8 h L:D photoperiod. Mortality was scored after seven days. The mean of mortality percentages and the SE were calculated using Graphpad Prism 7.0 software (La Jolla California, USA). The same software was used to compare the mortalities with a one-way analysis of variance (ANOVA). If the one-way ANOVA was significant (P < 0.05), mortality means were analyzed using the Tukey test, considering them significantly different when P < 0.05.
Production, solubilization and activation of Cry proteins
The production and further solubilization of the Bt crystal proteins was performed as described by Estela et al. (2004). The solubilized protoxins obtained were mixtures of different non-activated, partially-activated, and activated forms. The full protein activation was carried out with 10% trypsin (w/w) for 2 h at 37 °C. The solubilized protoxins and trypsin activated proteins were analyzed by sodium dodecyl sulphate 12% polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli 1970). Protein concentrations were determined by Bradford assay (Bradford 1976).
Insect cells lines and cell viability assays
Four insect cell lines were used in this study: BTI-Tn-5B1-4 (Hi5) from ovarian cells of cabbage looper, Trichoplusia ni (Granados et al. 1994), RP-HzGUT-AW1 (HzGUT) from gut cells of Helicoverpa zea (Goodman et al. 2004), UCR-SE from beet armyworm, S. exigua (Gelernter and Federici 1986) and Sf21 cells from ovaries of the fall armyworm, S. frugiperda (Vaughn et al. 1977). HzGUT and UCR-SE cells were grown in Excell insect cell culture medium (Sigma-Aldrich) supplemented with 5% fetal bovine serum (FBS). Hi5 and Sf21 were maintained in TNM-FH medium (Sigma-Aldrich) with 5% FBS and Grace’s medium (Gibco® Life technologies) with 10% FBS, respectively. Insect cell lines were maintained under the conditions recommended by the supplier (Laboratory of Virology, Wageningen University, The Netherlands).
The cytocidal activity was assessed by microscopy and cell viability assays. Cells with clear morphological changes induced by toxins (swollen cells, balloon-shaped cells and lysed cells), were recognized with an inverted microscope (Leica DMI 3000B) and recorded as affected cells. For the assays, the number of cells was determined with a Countess Automated Cell counter (Invitrogen). Aliquots of 100 µl of cell suspension at a concentration of 2 × 106 cells ml−1 were seeded into 96-well plates and incubated at 25 °C for at least 2 h. For the single-dose assays, 10 µl of either solubilized protein (1 mg ml−1) or trypsin activated toxin (0.7 mg ml−1), were added into each well and cell viability was measured after 16 h. For dose–response assay, cells were treated with 10 µl of trypsinized protein at five different concentrations (from 0.64, 3.2, 16, 80 to 400 µg ml−1) and cell viability was measured after 6 h. After adding CellTiter 96® AQueous One Solution Reagent (Promega Co., Madison, WI, USA), cell viability was quantified by measuring the absorbance at 490 nm in a spectrophotometry plate reader (Tecan infinite Pro200). Negative controls were performed with protein buffer (50 mM Na2CO3 buffer, pH 10.5) and positive controls were performed with 0.2% Triton X-100. The percentages of viability were calculated as: [(Absorbancesample − Absorbancepositive control)/(Absorbancenegative control − Absorbancepositive control)] × 100. Three different experiments (each one with replicated points) were carried out for each protein and insect cell line. Taking into account the number of cells seeded on the plates and the percentages of viability obtained, the number of viable cells were calculated. The mean of the viable cells in each cell line tested with activated and non-activated crystal proteins were compared using an unpaired t test. Values were considered statistically different when P values were lower than 0.05. The assays performed with different protein concentrations were analyzed with non-linear regression (sigmoidal curve fitting) using Graphpad Prism 7.0 software. This analysis provides the parameters of the half-maximal effective concentration (EC50), with 95% confidence interval and the slope of the sigmoidal curve. EC50 represents the concentration of a compound where 50% of its maximal effect is observed. The slope describes the steepness of the dose–response curve. EC50 values were considered similar when overlapped 95% confidence intervals, and different when there was no overlapping.
Proteomic analysis
LC–MS/MS analysis were performed at the proteomics facility of the SCSIE (Servei Central de Suport a la Investigació Experimental), at the University of Valencia, Valencia, Spain. Briefly, samples were subjected to trypsin digestion and loaded onto a trap column (NanoLC Column, 3 µm C18-CL, 75 µm × 15 cm; Eksigent). The peptides obtained were loaded onto an analytical column (LC Column, 3 µm C18-CL, 75 µm × 12 cm, Nikkyo) and analyzed in a mass spectrometer nanoESI qQTOF (5600 TripleTOF, ABSCIEX). ProteinPilot v5.0. search engine (ABSciex) with default parameters was used to generate peak list. The Paragon algorithm of ProteinPilot was used to search NCBI and Expasy protein database. The LC–MS/MS results with the confidence of identification of at least 95% were considered significant. Redundant proteins obtained in the analysis were omitted, but proteins with high ProtScore values and proteins with several discriminating, distinct and specific peptides, were recorded.
β-exotoxin detection
The presence of β-exotoxin in seven Bt strains was also determined by LC–MS/MS. Sample preparation was done as described by Hernández et al. (2003). The standard β-exotoxin (type I) from Bt HD2 was kindly supplied by I. Thiéry (Institut Pasteur, France) and was used to obtain the calibration curve. It has been reported that β-exotoxin can be detected in Bt HD2 and B. thuringiensis subsp. thuringiensis VMA10.30 strains, but is absent in Bt HD1 strain (Hernández et al. 2003). Samples with absence of β-exotoxin prepared from Bt HD1 strain were used as negative controls and samples with β-exotoxin, prepared form Bt HD2 and VMA10.30 strains, were used as positive controls.
Gene content identification
The total DNA from the selected Bt strains was extracted and purified following Ferrandis et al. (1999). Bt strains were characterized by using primers designed in this work or previously described in the literature (Supplementary Table S1). PCR reactions were conducted in 25 µl reaction mixtures containing 1 µl of 100 ng µl−1 of DNA template, 1 U of taq DNA polymerase (Biotools B&M labs, S.A. Madrid, Spain), 2.5 µl of 10× standard reaction buffer with MgCl2 (50 mM MgCl2 solution), 0.4 µM of each primer and 0.2 mM of dNTPs, in an Eppendorf Mastercycler thermal cycler (Eppendorf AG, Barkhuasenweg, Germany). PCR conditions were: 5 min of initial denaturation at 94 °C followed by 30 cycles of amplification with 1 min of denaturation at 94 °C, 45–60 s of annealing at 45–67 °C (depending on each pair of primers), 1–2 min of extension at 72 °C and an extra extension step of 10 min at 72 °C. PCR products were analyzed by 1% agarose gel electrophoresis. The amplified DNA was purified using NucleoSpin Gel and PCR Clean-up kit (MACHEREY–NAGEL, Germany) and sent to Stab Vida (Investigação e Serviços em Ciências Biologicas Lda, Portugal) for sequencing. The sequences were edited and analyzed using Geneious software (version 10.0.9). The DNA sequence analyses including alignment and Basic Local Alignment Search Tool (BLAST; https://blast.ncbi.nlm.nih.gov) were performed using the NCBI database and Geneious software.
Results
B. thuringiensis strains characterization
The selection of Iranian Bt strains was performed according to the toxicity of 130 Bt strains against Indian meal moth, P. interpunctella, second instar larvae at a single discriminating concentration of 108 spores ml−1 (data not shown). Five Bt strains, IE-1, AzLp, IE-2, IP-2 and IEp, isolated from infected larvae and showing bipyramidal crystals, were highly toxic P. interpunctella (Table 1). Besides, KhF (spherical crystals) and RM (irregular crystals) strains respectively with low and non-toxic activity towards P. interpunctella (Table 1), were chosen to determine the specific properties of the five highly toxic ones.
A bioassay screening at a single concentration of protoxins and trypsin activated toxins was performed for the seven selected strains and for the Bt HD1 reference strain, using first instar larvae of S. exigua, G. molesta, M. brassicae and O. nubilalis (Table 1). As a result, G. molesta and O. nubilalis were the most susceptible insects. Various degrees of mortality were found in S. exigua and M. brassicae first instar larvae treated with the highly toxic strains. Among those, AzLp, IE-2 and IP-2 protoxins and activated toxins, exhibited higher levels of toxicity for S. exigua and M. brassicae compared to that of the reference strain, Bt HD1. Neither toxic activity nor growth inhibition was observed for KhF protoxins. The toxicity of the trypsinized proteins of KhF was not analyzed since it was not possible to solubilize the KhF crystals not even after trying different buffer compositions and pH conditions (data not shown).
To assess the possible toxicity of the strains due to β-exotoxin production, LC–MS/MS was also used to check whether the selected strains produce this metabolite. The results showed that none of the seven selected Bt strains produced type I β-exotoxin, similarly to what was found for the negative control strain Bt HD1.
Cell viability
The cytocidal spectrum of the selected Bt strains and the reference strain Bt HD1 was studied against four lepidopteran cell lines in terms of loss of cell viability. Figure 1 shows the results of cytocidal activities (% viability) of activated vs. non-activated proteins. In HzGUT cells, the cytocidal activities of activated and non-activated proteins were significantly different for all the Bt strains (P values from < 0.0001 to 0.0268), whereas in the Sf21 cell line no significant differences were found. In UCR-SE and Hi5 cell lines, significant differences in viability were observed when the cells were treated with protoxins or activated toxins of IE-2 (P values 0.0350 and 0.0004, respectively), and IEp (P values 0.0057 and < 0.001, respectively) strains, and IP-2 treatments also induced differences in UCR-SE (P-value = 0.0044). Differences in membrane associated proteases involved in Cry proteins activation could explain the results obtained from the analyzed cell lines.
Susceptibility of UCR-SE, HzGUT, Hi5 and Sf21 cells to protoxins (Pro) and trypsin activated proteins (Act) from the different Bt strains. Bars represent percentages of viability after 16 h of exposure to 1 mg ml−1 of protoxins or 0.7 mg ml−1 of activated toxins. SE of the results from three different experiments were represented in each bar. (*) Statistically significant differences between protoxin and activated protein treatments (t test): in HzGUT cell line; HD-1 (t = 18.6, df = 4, P < 0.0001), IE-1 (t = 254.6, df = 4, P < 0.0001), AzLp (t = 3.4, df = 4, P-value = 0.0268), IE-2 (t = 12.7, df = 4, P-value = 0.0002), IP-2 (t = 6.0, df = 4, P-value = 0.0039), IEp (t = 8.6, df = 4, P-value = 0.0010) and RM (t = 18.0, df = 4, P < 0.0001). In UCR-SE cell line; IE-2 (t = 3.1, df = 4, P-value = 0.0350), IP-2 (t = 5.8, df = 4, P-value = 0.0044) and IEp (t = 5.4, df = 4, P-value = 0.0057). In Hi5 cell line; IE-2 (t = 11.2, df = 4, P-value = 0.0004) and IEp (t = 18.7, df = 4, P-value < 0.0001)
In general, proteins from Bt HD1 showed similar toxicity to all cell lines (94% mean value of cells viability). Proteins from AzLp, IE-2 and IP-2 Bt strains were highly cytocidal for the four tested cell lines with 60–100% cell viability reduction (Fig. 1). Indeed, after 16 h cell exposure, no Sf21 viable cells could be observed. The least toxic for all the tested cell lines were proteins from IE-1 and RM strains (92% mean value of cells viability). The toxicity of IEp protoxins to all cell lines was similar (92% mean value of cells viability), while the toxicity of IEp tripsinized toxins ranged from 24 to 93%.
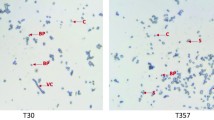
Sf21 cell line was employed for further investigation due to its high susceptibility to Bt toxins. The results of the dose–response assays with trypsin activated proteins of AzLp, IE-2 and IP-2 strains, revealed that the viability of Sf21 cells decreased with the increase of trypsin activated toxin concentrations. Osmotic swelling and balloon-shaped cells were observed after 3 h exposure of the cells to a mid-protein concentration (16 µg ml−1) and lysis was observed when cells were exposed to highest concentrations (Fig. 2). The statistical analyses showed that EC50 values of AzLp and IE-2 were different (Table 2).
Protein analyses
The protein patterns of the parasporal crystals of the seven selected Bt strains were obtained by SDS-PAGE electrophoreses. The SDS-PAGE profiles of the standard strain Bt HD1 and the selected strains were similar, with protein bands of molecular weights between 60 and 130 kDa (Fig. 3a). As it is known, Bt HD1 produces Cry1Aa, Cry1Ab, Cry1Ac and Cry2Aa proteins (Schnepf et al. 1998). This composition fits with the occurrence of two main bands of about 130 kDa (which would correspond to Cry1 protoxins) and 65 kDa (which would correspond to Cry2 protoxins). The presence of other bands between 130 and 60 kDa can be due to partial processing of the main proteins. Similarly to Bt HD1, our strains contained a protein band of around 130 kDa in size, which could correspond to Cry1 but also to Cry7, Cry8 or Cry9-type proteins (Arrieta et al. 2004). Parasporal inclusions from strains Bt HD1, IE-1, IP-2 and IEp showed a distinct additional band of 65–75 kDa that could correspond to Cry2, Cry3 or Cry22-type proteins. After solubilization and trypsin activation, the protein profiles were almost identical for all strains and showed bands of about 60 kDa (Fig. 3b), a common molecular weight of activated Cry toxins.
The insecticidal crystal proteins produced by each one of the Bt strains were analyzed by LC–MS/MS. The proteins were retrieved from the row data following the criteria specified in Materials and Methods and were further validated by using BLAST. The detected proteins for each Bt strains were sorted according to their relative abundancy based on the numbers of distinct peptides found (having at least 95% confidence) for a specific protein (Supplementary Table S2). The final analysis showed that the proteins present in the solubilized crystal protein mixtures of the selected Bt strains were Cry1Aa, Cry1Ab, Cry1Ac, Cry1Ca, Cry1Da and Cry2Aa (Table 3). The proteins from the Cry1A family were the most abundant ones according to quantitative values. Cry1Ac was the only protein assigned to RM strain. For KhF strain, no protein similar to the known Bt insecticidal crystal proteins were identified. Probably, either the insecticidal crystal proteins in KhF strain were under the detectable level of Paragon algorithms employed, or the KhF parasporal crystals contain new insecticidal crystal proteins.
PCR screening of insecticidal Bt coding genes
PCR analysis was carried out to identify genes encoding proteins secreted during vegetative phase of Bt growth (such as cry1I, vip1, vip2, vip3 and sip1), as well as genes encoding proteins that accumulate in crystals produced during the sporulating phase, of the cry, cyt and ps gene families. The amplification of cry2 gene was observed in all tested strains. Conversely, none of the strains showed amplification products for cry1Ad, cry1Ag, cry1G, cry1H, cyt1, cyt2, vip1, vip2, sip1, ps1, ps2, ps3 and ps4 genes.
Strains IE-1, AzLp, IE-2, IP-2 and IEp showed positive amplification of the predicted band size for cry1Ac, cry1I, vip3 genes. The cry1Aa gene was present in IE-1, AzLp, IE-2 and IP-2. The cry1C and cry1D genes were found in three strains: AzLp, IE-2 and IP-2 (Table 3).
Discussion
In this study, the characterization of seven Iranian Bt strains has been investigated, on the basis of lepidopteran toxic activity spectrum, in vivo and in vitro toxicity assays, protein profiling, proteomics analysis, gene content identification and β-exotoxin production. The P. interpunctella most toxic strains produced bipyramidal crystals. It has been described that the toxic Bt proteins for P. interpunctella are Cry1Aa, Cry1Ab, Cry1Ac, Cry1Ad, Cry1B, Cry1C, Cry1D, Cry1E, Cry1F, Cry1I, Cry2A, Cry9, Cry39 and Cry40, (Gryspeirt and Gregoire 2012; Shojaaddini et al. 2012). The Cry protein contents identified by LC–MS/MS analysis in our study showed that the two strains causing 100% mortality to P. interpunctella (IP-2 and IEp) have completely different protein contents. The comparison of toxicity and proteomics analysis indicates that not only composition but also the relative abundance of the expressed proteins in each strain is important for toxicity. This statement is reinforced if we compare the proteins of IEp (100% toxicity and Cry1Ac and Cry2Aa as major proteins in the crystals), IE-1 (67% mortality with Cry1Ac, Cry1Aa and Cry2Aa as major proteins in the crystals) and RM (low toxicity, producer of only Cry1Ac similarly to Bt subsp. kurstaki HD-73 (Ibrahim et al. 2010)).
In general, the proteomic analysis and PCR results were consistent in the sense that when a protein was detected in a strain, the corresponding gene was detected by PCR. This correspondence did not always happen in the opposite direction. Indeed, the cry2 gene was amplified in all tested strains but the protein was only detected in IE-1 and IEp. Also the cry1Ac gene was detected in IE-2 and IP-2 but the protein was not detected in the LC–MS/MS analyses. This could be explained by a low transcription rate of the gene for diverse reasons, or due to the presence of defective untranscribed sequences. In conclusion, the PCR analyses, widely used in Bt screenings, can be useful solely to predict the proteins that may compose the Bt crystal.
Genes of the cry1 family were found in all strains, excluding KhF, in accordance with the presence of the major band of about 130 kDa in SDS-PAGE and with the proteomic analyses. In the previous reports (Uribe et al. 2003; Thammasittirong and Attathom 2008), cry1G was considered as an abundant gene in the Bt collections, contrarily to our results. On the other hand, in the Chinese (Wang et al. 2003) and Iranian (Seifinejad et al. 2008) Bt collection, cry1G and cry1H were found rarely in agreement with our results. Therefore, the presence of these genes could be related with the origin of the strains. In the present work, the cry2 gene was detected in all strains, in agreement with several studies that report the high frequency of cry2 genes, especially among cry1 containing strains (Porcar and Juárez-Pérez 2003; Seifinejad et al. 2008). Regarding vip and sip genes, the frequencies found in this and other screening studies differ or not depending on the origin of the samples analyzed (Salehi Jouzani et al. 2008; Seifinejad et al. 2008; Hernandez-Rodriguez et al. 2009; Djenane et al. 2017).
The toxicity spectrum of second series of bioassays was restricted to four different lepidopteran families, namely Noctuidae, Tortricidae, Crambidae and Pyralidae. As a result, three Bt strains, AzLp, IE-2 and IP-2, showed higher toxicity against S. exigua and M. brassicae first instar larvae than the standard Bt HD1 (Table 1). This was in accordance with the loss of viability observed in the UCR-SE cells exposed to the activated toxins of AzLp, IE-2 and IP-2 strains (Fig. 1). The toxicity of these strains on S. exigua could be due to the presence of Cry1C and Cry1D proteins (Hernández-Martinez et al. 2008). Mamestra brassicae first instar larvae are susceptible to Cry1C (Gilliland et al. 2002), and accordingly, the highest mortality was recorded in the Cry1C protein producers, AzLp, IE-2 and IP-2 strains (Tables 1, 3).
Grapholita molesta was the most susceptible insect in this study, due to the high toxic effect of Cry1Aa, Cry1Ac and Cry1C proteins against this pest (Scaramal Ricietto et al. 2016). As reported in other studies (Hernández-Martinez et al. 2008) Cry1Ac is not toxic for S. exigua, which would be in agreement with the absence of toxicity exhibited by RM, a strain that following our proteomics and PCR results only possesses and express cry1Ac gene (Table 3). There are several studies that report susceptibility of O. nubilalis to Cry1Aa, Cry1Ab, Cry1Ac, Cry1Fa, Cry1Ie2, Cry9Ca and Cry9E (van Frankenhuyzen 2009; Zhao et al. 2015). Regarding the presence of Cry1Aa, Cry1Ab and Cry1Ac in the Bt strains studied, high susceptibility of O. nubilalis was expected and indeed observed (Table 1).
To expand our knowledge of the insecticidal activities of the selected strains, four lepidopteran derived cell lines were used. It has been discussed that the response of cell lines to different Bt toxins is not necessarily correlated with the in vivo activity toward the insect from which the cells are derived (Witt et al. 1986). To test this, the toxicity of our Bt strains towards S. exigua first instar larvae and the S. exigua cell line UCR-Se was assessed and compared. Our results showed high correlation between bioassays and cell viability assays (Table 1 and Fig. 1), pointing to the feasibility of using insect cell lines for toxicity screening.
In this study, the cell viability assays indicated that the crystal proteins from AzLp, IE-2 and IP-2 (the three most toxic strains in bioassays) were highly toxic for H. zea, T. ni, S. exigua and S. frugiperda cells. This is in accordance to what has been described for Sf21 and Hi5 cells lines, highly susceptible to Cry1C toxins (Kwa et al. 1998; Gringorten et al. 1999). According to our cell viability assay, Sf21, UCR-SE, Hi5 and HzGUT were not susceptible to IE-1 and RM, strains that mainly produced Cry1Ac protein. This is in agreement with Gringorten et al. (1999) and Chen et al. (2015) that reported no susceptibility for Sf9, Sf21 and Hi5 cells to Cry1Aa, Cry1Ab and Cry1Ac proteins. Cry1Ac and Cry1F are toxic for heliothines (Siebert et al. 2008), in contrast to our cell viability assay results, that showed low susceptibility of HzGUT to IE-1, IEp and RM strains, in which the cry1Ac gene was expressed. These results point out that cell cultures do not always show the toxicity responses expected based on the bioassays performed on the insects from which they originally derived.
The results of the bioassay with the protoxins of the KhF strain indicated that this strain was almost not toxic for the tested insects. The PCR technique only detected cry2 genes and proteomics did not reveal any known Cry protein. But KhF spore and crystal mixture had been moderately toxic for second instar larvae of P. interpunctella. These results can be due to the presence of immune inhibitors and Bacillolysin as bioactive factors, which had been detected in KhF strain by LC–MS/MS analysis (data not shown).
In summary, to predict the insecticidal potency of newly isolated Bt strains, the LC-MS/MS analysis (which assess crystal protein composition) is a useful technique much more convenient than PCR-based characterization which can only offer a prediction of the proteins composing the crystal. But bioassays (with insects or with cell lines) are crucial and unavoidable steps to complete the strains characterization, since protein proportion and protein interaction between different toxins seem to be decisive for toxicity. As a result, due to the high toxicity towards different lepidopteran insect larvae and cell lines, the presence of the insecticidal proteins Cry1Aa, Cry1Ab, Cry1Ac, Cry1Ca and Cry1Da, and the absence of β-exotoxin, AzLp, IE-2 and IP-2 Bt strains could be considered as important candidates for development of future Bt based insecticides.
References
Arrieta G, Hernández A, Espinoza AM (2004) Diversity of Bacillus thuringiensis strains isolated from coffee plantations infested with the coffee berry borer Hypothenemus hampei. Rev Biol Trop 52:757–764
Bel Y, Granero F, Alberola TM, Martínez-Sebastián MJ, Ferré J (1997) Distribution, frequency and diversity of Bacillus thuringiensis in olive tree environments in Spain. Syst Appl Microbiol 20:652–658
Bel Y, Ferré J, Escriche B (2011) Quantitative real-time PCR with SYBR Green detection to assess gene duplication in insects. BMC Res Notes 4:84
Berόn CM, Salerno GL (2006) Characterization of Bacillus thuringiensis isolates from Argentina that are potentially useful in insect pest control. BioControl 51:779–794
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of dye-binding. Anal Biochem 72:248–254
Bravo A, Likitvivatanavong S, Gill SS, Soberón M (2011) Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochem Mol Biol 41:423–431
Chen Z, He F, Xiao Y, Liu C, Li J, Yang Y, Ai H, Peng J, Hong H, Liu K (2015) Endogenous expression of a Bt toxin receptor in the Cry1Ac-susceptible insect cell line and its synergistic effect with cadherin on cytotoxicity of activated Cry1Ac. Insect Biochem Mol Biol 59:1–17
Djenane Z, Nateche F, Amziane M, Gomis-Cebolla J, El-Aichar F, Khorf H, Ferré J (2017) Assessment of the antimicrobial activity and the entomocidal potential of Bacillus thuringiensis isolates from Algeria. Toxins 9:139–158
Estela A, Escriche B, Ferré J (2004) Interaction of Bacillus thuringiensis toxins with larval midgut binding sites of Helicoverpa armigera (Lepidoptera: Noctuidae). Appl Environ Microbiol 70:1378–1384
Ferrandis MD, Juárez-Pérez VM, Frutos R, Bel Y, Ferré J (1999) Distribution of cryl, cryll and cryV genes within Bacillus thuringiensis isolates from Spain. Syst Appl Microbiol 22:179–185
Ferré J, Escriche B, Bel Y, Van Rie J (1995) Biochemistry and genetics of insect resistance to Bacillus thuringiensis insecticidal crystal proteins. FEMS Microbiol Lett 132:1–7
Gelernter WD, Federici BA (1986) Continuous cell line from Spodoptera exigua (Lepidoptera: Noctuidae) that supports replication of nuclear polyhedrosis viruses from Spodoptera exigua and Autographa californica. J Invertebr Pathol 48:199–207
Gilliland A, Chambers CE, Bone EJ, Ellar DJ (2002) Role of Bacillus thuringiensis Cry1 δ endotoxin binding in determining potency during lepidopteran larval development. Appl Environ Microbiol 68:1509–1515
Gomis-Cebolla J, Ruiz de Escudero I, Vera-Velasco NM, Hernández-Martínez P, Hernández-Rodríguez CS, Ceballos T, Palma L, Escriche B, Caballero P, Ferré J (2017) Insecticidal spectrum and mode of action of the Bacillus thuringiensis Vip3Ca insecticidal protein. J Invertebr Pathol 142:60–67
Goodman CL, Wang AA, Nabli H, McIntosh AH, Wittmeyer JL, Grasela JJ (2004) Development and partial characterization of heliothine cell lines from embryonic and differentiated tissues. In Vitro Cell Dev Biol Anim 40:89–94
Granados RR, Li GX, Derksen ACG, McKenna KA (1994) A new insect cell line from Trichoplusia ni (BTI-Tn-5B1-4) susceptible to Trichoplusia ni single enveloped nuclear polyhedrosis virus. J Invertebr Pathol 64:260–266
Gringorten JL, Sohi SS, Masson L (1999) Activity spectra of Bacillus thuringiensis δ-endotoxins against eight insect cell lines. In Vitro Cell Dev Biol Anim 35:299–303
Gryspeirt A, Gregoire JD (2012) Effects of two varieties of Bacillus thuringiensis maize on the biology of Plodia interpunctella. Toxins 4:373–389
Hernández CS, Martínez C, Porcar M, Caballero P, Ferré J (2003) Correlation between serovars of Bacillus thuringiensis and type I β-exotoxin production. J Invertebr Pathol 82:57–62
Hernández-Martinez P, Ferré J, Escriche B (2008) Susceptibility of Spodoptera exigua to 9 toxins from Bacillus thuringiensis. J Invertebr Pathol 97:245–250
Hernández-Rodriguez CS, Boets A, van Rie J, Ferré J (2009) Screening and identification of vip genes in Bacillus thuringiensis strains. J Appl Microbiol 107:219–225
Ibrahim MA, Griko N, Junker M, Bulla LA (2010) Bacillus thuringiesis a genomics and proteomics perspective. Bioeng Bugs 1:31–50
Juárez-Pérez VM, Ferrandis MD, Frutos R (1997) PCR-based approach for detection of novel Bacillus thuringiensis cry gene. Appl Environ Microbiol 63(8):2997–3002
Kwa MSG, de Maagd RA, Stiekema WJ, Vlak JM, Bosch D (1998) Toxicity and binding properties of the Bacillus thuringiensis delta-endotoxin Cry1C to cultured insect cells. J Invertebr Pathol 71:121–127
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
McGaughey WH, Beeman RW (1988) Resistance to Bacillus thuringiensis in colonies of Indian meal moth and almond moth (Lepidoptera: Pyralidae). J Econ Entomol 81:28–33
Ohba M, Mizuki E, Uemori A (2009) Parasporin, a new anticancer protein group from Bacillus thuringiensis. Anticancer Res 29:427–433
Porcar M, Juárez-Pérez VM (2003) PCR-based identification of Bacillus thuringiensis pesticidal crystal genes. FEMS Microbiol Rev 26:419–432
Salehi Jouzani G, AbadAP Seifinejad A, Marzban R, Kariman K, Maleki B (2008) Distribution and diversity of dipteran-specific cry and cyt genes in native Bacillus thuringiensis strains obtained from different ecosystems of Iran. J Ind Microbiol Biotechnol 35:83–94
Scaramal Ricietto AN, Gomis-Cebolla J, Vilas-Boas GT, Ferré J (2016) Susceptibility of Grapholita molesta (Busck, 1916) to formulations of Bacillus thuringiensis, individual toxins and their mixtures. J Invertebr Pathol 141:1–5
Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J (1998) Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev 62:775–806
Seifinejad A, Salehi Jouzani G, Hosseinzadeh A, Abdmishani C (2008) Characterization of Lepidoptera-active cry and vip genes in Iranian Bacillus thuringiensis strain collection. Biol Control 44:216–226
Shojaaddini M, López MJ, Moharramipour S, Khodabandeh M, Talebi AA, Vilanova C, Latorre A, Porcar M (2012) A Bacillus thuringiensis strain producing epizootics on Plodia interpunctella: a case study. J Stored Prod Res 48:52–60
Siebert MW, Nolting S, Leonard BR, Braxton LB, All JN, van Duyn JW, Bradley JR, Bacheler J, Huckaba RM (2008) Efficacy of transgenic cotton expressing Cry1Ac and Cry1F insecticidal protein against heliothines (Lepidoptera: Noctuidae). J Econ Entomol 101:1950–1959
Thammasittirong A, Attathom T (2008) PCR-based method for the detection of cry genes in local isolates of Bacillus thuringiensis from Thailand. J Invertebr Pathol 98:121–126
Uribe D, Martinez W, Ceron J (2003) Distribution and diversity of cry genes in native strains of Bacillus thuringiensis obtained from different ecosystems from Colombia. J Invertebr Pathol 82:119–127
van Frankenhuyzen K (2009) Insecticidal activity of Bacillus thuringiensis crystal proteins. J Invertebr Pathol 101:1–16
Vaughn JL, Goodwin RH, Tompkins GJ, McCawley P (1977) The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro 13:213–217
Wang JH, Boets A, Van Rie J, Ren GX (2003) Characterization of cry1, cry2 and cry9 genes in Bacillus thuringiensis isolated from China. J Invertebr Pathol 82:63–71
Willcoxon MI, Dennis JR, Lau SI, Xie W, You Y, Leng S, Fong RC, Yamamoto T (2016) A high-throughput, in-vitro assay for Bacillus thuringiensis insecticidal proteins. J Biotechnol 217:72–81
Witt DP, Carson H, Hodgdon JC (1986) Cytotoxicity of Bacillus thuringiensis δ-endotoxins to cultured Cf-1 cells does not correlate with in vivo activity towards spruce budworm larvae. In: Samson RA, Vlak JM, Peters D (eds) Fundamental and applied aspects of invertebrate pathology. Academic Press, San Diego, pp 3–6
Zhao C, Jurat-Fuentes JL, Abdelgaffar HM, Pan H, Song F, Zhang J (2015) Identification of new cry1I-type gene as a candidate for gene pyramiding in corn to control Ostrinia species larvae. Appl Environ Microbiol 81:3699–3705
Acknowledgements
This work is a part of PhD dissertation of the first author, which was financially supported by University of Tehran (Grant Ref. 73132800.6.22). We are deeply grateful to Dr. Patricia Hernández-Martínez for her valuable comments. We thank Rosa Maria González-Martínez for her support in laboratory assistance and Oscar Marin Vázquez for his help with insect rearing. Research was supported by the Spanish Ministry of Science and Innovation (Grant Ref. AGL2015-70584-C2-1-R), by a Grant of the Generalitat Valenciana (Grant Ref. GVPROMETEOII-2015-001) and by European FEDER funds. We are very grateful to Luz Valero, at the Proteomics Unit of the SCSIE (Servei Central de Suport a la Investigació Experimental), at the University of Valencia (a member of the ISCIII ProteoRed Proteomics Platform) for her helpful comments and discussions on proteomics results.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Nicolai Meyling.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khorramnejad, A., Talaei-Hassanloui, R., Hosseininaveh, V. et al. Characterization of new Bacillus thuringiensis strains from Iran, based on cytocidal and insecticidal activity, proteomic analysis and gene content. BioControl 63, 807–818 (2018). https://doi.org/10.1007/s10526-018-9901-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-018-9901-9