Abstract
Saltcedars are woody plants in the genus Tamarix L. (Caryophyllales: Tamaricaceae) and are native to Eurasia and Africa. Several species have become invasive in the Americas, Australia and South Africa. In Argentina there are four species of Tamarix distributed in arid, semi-arid and coastal areas of most provinces. The taxonomic isolation of Tamarix spp. in Argentina, their widespread distribution, negative impact to natural areas and lack of impact from existing natural enemies all indicate that Tamarix is an ideal candidate for classical biological control in Argentina. Biological control of Tamarix spp. has been rapid and highly successful in the USA after the introduction of four Diorhabda spp. (Coleoptera: Chrysomelidae). Biological control of Tamarix spp. in Argentina could be implemented easily, rapidly, and at a low cost by utilizing the information developed in the USA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive exotic plants constitute threats to economic activity and human welfare (Mack et al. 2000), and are also one of the greatest threats to rare, endangered, and threatened species (Simberloff et al. 2013). Exotic trees are among the most widespread and damaging invasive species (Richardson and Rejmánek 2011). In the past few decades invasive trees have escalated in importance worldwide due to the rapid increase in the human-mediated transport and dissemination of thousands of species for a wide range of purposes namely, forestry, agroforestry, ornamental horticulture and food production (Richardson et al. 2014). According to the National System on Information on Exotic Invasive Species of Argentina (INBIAR-UNS 2016), there are 90 invasive alien shrubs and trees in Argentina, many of which cause major ecological and economic problems. Among these, saltcedars, Tamarix spp. (Caryophyllales: Tamaricaceae), are widely colonizing riparian and wetlands systems in arid and semi-arid inland and coastal areas of Argentina (Natale et al. 2008, 2013; APN-SIB 2016). Native to Eurasia and Africa, several Tamarix spp. have also become invasive weeds in USA, Mexico, Australia and South Africa after their introduction for ornamental, windbreak, shade tree, sand dune stabilization, and erosion control (Pasiecznik 2016). Environmental changes associated with Tamarix proliferation in invaded areas include water course modifications, recruitment within riverbeds that can block the flow of rivers and cause flooding events, displacement of native vegetation, soil salinization and increased fire frequency (DeLoach et al. 2000).
In Argentina, although Tamarix seem to be at early stages of their invasion process, there remains the potential to invade more than three quarters of the arid and semi-arid areas of the country, constituting one of the main threats to biodiversity (Natale et al. 2013). Tamarix is reported as an invasive weed in several protected natural areas of Argentina (APN-SIB 2016), including the Llancanelo and Guanacache lakes, two RAMSAR sites in the Cuyo region of central-western Argentina (FAO GEF 2017; INBIAR-UNS 2016). Due to the socio-economic impact and effect on biodiversity, the Tamarix species complex has been prioritized as one of the six pilot programs of the National Strategy on Invasive Alien Species (NSIAS) (FAO GEF 2017).
Damage from invasive species cannot easily be reversed, and once established, invaders often persist indefinitely and spread to their ecological limits (van Driesche et al. 2010). Current methods to limit the continued spread of Tamarix in Argentina rely on mechanical removal and chemical treatments (Sosa et al. 2006; Natale et al. 2014). However, to achieve Tamarix suppression or prevent expansion at the landscape level, classical biological control (CBC) should be considered and integrated with other control approaches. Weed CBC constitutes an ecologically sound, cost effective, and sustainable control option as a component of an integrated pest management program (van Driesche et al. 2010).
Biological control of Tamarix spp. has been rapid and highly successful in the USA after the introduction of four Diorhabda spp. (Coleoptera: Chrysomelidae) (Pattison et al. 2011; Hultine et al. 2015; DeLoach et al. 2014). When a CBC project has been successful in one country, the costs associated with the exploratory surveys, risk assessments and rearing procedures of suitable biological control agents are considerably reduced in other countries. Transferring this technology from one country to another has been defined as the “fast track” or “short route” (Harley and Forno 1992). As a consequence, biological control of Tamarix spp. in Argentina could be expedited and developed at a lower cost by utilizing the information developed in the USA.
The Diorhabda spp. introduced into the USA may share similarly narrow host ranges but they respond differently to environmental conditions (Milbrath and DeLoach 2006; Dalin et al. 2010), latitude (Bean et al. 2007) and invertebrate predation (Knutson et al. 2014) that will likely influence the establishment, spread, and success of Diorhabda spp. in suppressing Tamarix in Argentina. To investigate environmental factors, Maximum Entropy Species Distribution Model (MaxEnt) was developed to assess the suitability of Argentina’s climate for colonization by Diorhabda spp., their potential distribution and resulting overlap of the herbivores and their host T. ramosissima Ledeb. (Phillips et al. 2006).
The objective of this work is to examine the suitability of Tamarix spp. as a target for CBC in Argentina. Here we present and discuss factors relating to CBC feasibility, including: (1) taxonomy and geographic distribution, (2) environmental and economic damage (3) available control methods, (4) uses of Tamarix in Argentina and potential conflicts of interest (5) potential for classical biological control of Tamarix spp. in Argentina and (6) suitability of Argentina’s climate for colonization by Diorhabda species.
Taxonomy and geographic distribution
The Tamaricaceae are found in temperate and subtropical regions of Africa and Eurasia (Stevens 2001). This family comprises about 100 species in five genera: Hololachna Ehrenb., Myricaria Desv., Reaumuria L., Myrtama Ovcz. & Kinzik., and Tamarix L., which is the largest genus containing 54 species (Crins 1989). Originating from western China, Tamarix has radiated to Mongolia, Korea, India, and across the Middle East to the eastern Mediterranean area, southern Europe, and northeastern and southern Africa (Crins 1989; Pasiecznik 2016). Although no Tamarix species occur naturally in the western hemisphere or in Australia, a few species have become naturalized and invasive in deserts and semi-arid areas of North America, Australia, South Africa and South America (Pasiecznik 2016).
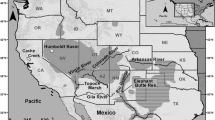
In Argentina, Tamarix occurs along a wide range from 49°14′11″ to 23°26′17″S, and from 70°35′45″ to 56°59′46″W (Fig. 1). To date, four species of Tamarix are found in Argentina, namely: T. gallica L., T. ramosissima Ledeb., T. chinensis Lour. and T. parviflora DC. (Natale et al. 2008). Hybridization between T. ramosissima and T. chinensis has been reported in their invaded ranges in USA (Gaskin and Kazmer 2009). However, in Argentina, this phenomenon has, so far, not been reported (Natale et al. 2012). The first three species colonize natural and semi-natural habitats, although T. ramosissima has the highest number of invading populations and is considered to be the most aggressive species (Natale et al. 2008).
Distribution of Tamarix spp. in Argentina (from Natale et al. 2008)
The Tamaricaceae have been historically considered the sister family of the Frankeniaceae for sharing morphological characters and secondary chemistry (Gaskin et al. 2004). Both families are placed in the order Caryophyllales (Stevens 2001) (Fig. 2). The Frankeniaceae is a small family of four genera and ca. 80 species of small halophytic shrubs or herbs of mostly temperate distribution, where Frankenia is the largest genus and the only genus of the family that is native to the western hemisphere (Whalen 1987). Ten species of Frankenia occur in southern South America, seven of which occur in Argentina (Zuloaga and Morrone 2009).
Phylogenetic tree of the Order Caryophyllales (http://www.mobot.org/mobot/research/apweb/orders/Caryophyllalesweb.htm). * Denotes nodes with 50–80% bootstrap support; unmarked nodes have > 80% support
Environmental and economic damage
The effects of Tamarix invasions, specifically of T. ramosissima, have been well-documented in riparian areas (Shafroth et al. 2005). In western USA, Tamarix is considered one of the most dominant complexes of woody species, occupying 470,000–650,000 ha across 23 western states (Zavaleta 2000). Tamarix roots bind together gravel and cobble riverbeds, resulting in enlarged bars and narrowed channels that increase the likelihood of floods (Cooper et al. 2003). Tamarix negatively affects the structure and function of riparian ecosystems by affecting habitat quality for wildlife, reducing biodiversity and altering food webs (Zavaleta 2000; Shafroth et al. 2005). Tamarix spp. have displaced native plant communities and may be a major contributor to the decline of many native plants and animals, including endangered species (Dudley and DeLoach 2004). Other effects of Tamarix invasions include limiting recruitment of native riparian trees (e.g. Populus and Salix species) through increased salinity (Dudley and DeLoach 2004) and suppression of arbuscular mycorrhizal fungal communities (Beauchamp et al. 2005). The leaf litter and foliage produced by T. ramosissima is flammable and encourages the spread of wildfires (DeLoach et al. 2000). Native vegetation and wildlife is destroyed in these fires, while T. ramosissima seedlings are able to increase their spread due their ability to re-sprout more successfully than native plants following fire (Drus et al. 2012).
Tamarix thickets can cause increased water loss in riverine systems via transpiration (Shafroth et al. 2005). In arid areas of the western USA, losses of stream-flow and ground water caused by Tamarix are estimated at US$133 to $285 million annually (Zavaleta 2000). Other economic losses include reduced utilization of parks and natural areas by hunters, fishermen, campers, bird watchers, wildlife photographers and others (Pasiecznik 2016). Losses due to non-consumptive, recreational uses in Arizona and New Mexico were estimated at US$29.5 and US$15.8 million per annum, respectively (Pasiecznik 2016).
There is no estimation of the economic impact caused by invasive alien species in Argentina (FAO GEF 2017). However, developing countries in general, like Argentina, are particularly vulnerable to the threats posed by invasive species. Invasive species can have a disproportionate impact on the biodiversity and economies of developing countries because these countries typically rely more heavily on agriculture, forestry and fishing than more developed countries, and have fewer resources to combat them (GISP 2007). In Argentina, Tamarix spp. affect the social and economic value of both highly productive land and subsistence agriculture areas (Natale et al. 2010). Also, the Tamarix spp. threaten biodiversity and conservation in dry ecosystems, including national parks and several protected areas, because of these species’ superior capacity to access and take up groundwater (FAO GEF 2017; APN-SIB 2016). At the RAMSAR site of Llancanelo Lake Reserve in the Cuyo region, Tamarix affects soil salinization, hydrological changes, loss of wild habitat and nesting sites for aquatic birds (Sosa et al. 2006).
Uses of Tamarix in Argentina and potential conflicts of interest
Species of Tamarix are mainly used as ornamentals, windbreaks, shade trees and for dune fixation along the coasts (Hurrell et al. 2004; Karlin et al. 2010). Tamarix spp. flowers constitute a nectar source for honeybees and are used as an astringent for treating diarrhea and other diseases (Hurrell et al. 2004; Karlin et al. 2010). Tender branches and leaves provide forage to livestock particularly during the dry period (Karlin et al. 2010). Tamarix wood is used as fuel and to construct fences and cattle pens (Hurrell et al. 2004; Karlin et al. 2010).
Biological control of Tamarix resulted in tree mortality and facilitated the recruitment of other native plants in large areas of the western USA after the introduction of four species of Diorhabda beetles (Table 1) (Pattison et al. 2011; Hultine et al. 2015; DeLoach et al. 2014). However, the rate of weed suppression has raised some concerns in terms of conflicts of interest between land managers seeking control of the exotic plant and conservationists who seek to protect the southwestern willow flycatcher (Empidonax traillii extimus) (SWF), an endangered subspecies using this non-native plant as nesting habitat in some western riparian ecosystems (Dudley and DeLoach 2004; Sogge et al. 2008). The primary concern of USA wildlife agencies was that Tamarix defoliation by the Diorhabda biocontrol agents could reduce tamarisk canopy cover over the nest site, exposing the southwestern willow flycatcher birds in the nests to excessive heating and desiccation, particularly because this species breeds relatively late in the season (Sogge et al. 2008; Dudley and Bean 2012).
In Argentina, the crowned solitary eagle (CSE, Buteogallus coronatus), which inhabits semi-arid environments in the north central part of the country, is listed as one of the 18 endangered bird species (BirdLife International 2017) and was documented to nest on Tamarix gallica (Maceda 2007). The CSE nest is a large platform of sticks located on the top of larger trees with good access from the air (Maceda 2007). Nest records of CSE include an important diversity of native (e.g. Prosopis, Aspidosperma Phyllostylon, Schinopsis) as well as exotic trees (e.g. Eucaliptus sp. Ulmus pumila) and man-made structures (Maceda 2007).
Considering the plasticity of the CSE to building its nests, it seems unlikely that Tamarix bioncontrol using Diorhabda beetles and CSE conservation represents a conflict of interest in Argentina. However, the controversy between Tamarix biocontrol and the SWF in the USA emphasizes the need for a restoration plan to mitigate possible negative consequences of exotic plant decline when passive recruitment of native species is slower than desired (Dudley and Bean 2012). The Tamarix control pilot program within the NSIAS in Argentina foresees the implementation of mitigation measures by recruiting native plant species in the Tamarix control areas (FAO GEF 2017). In addition, installation of artificial platforms could also be considered as a tool for management and conservation of the CSE if the birds are reluctant to roost on defoliated plants.
Control methods
Many efforts have been committed in recent decades to control Tamarix in the western USA, where it is an important invasive alien weed of floodplains and wetlands (Dudley and DeLoach 2004). Conventional control methods for Tamarix, i.e. mechanical removal and chemical treatments, benefited native species in numerous locations, and promoted a return of surface flows in some cases (Egan 1997). Yet the benefits of these approaches did not provide a permanent control of the problem as Tamarix often grew back or reinvaded from surrounding areas (Carruthers et al. 2006). In addition, such control methods incur high financial expenditures and entail collateral damage to associated aquatic resources and non-target native riparian vegetation (Dudley and DeLoach 2004). Also, these labor and technology-intensive approaches are difficult to apply in remote or inaccessible habitats, and treated sites exhibit a high frequency of re-infestation (Shafroth et al. 2005). Hand removal by pulling seedlings effectively kills the plants and causes little damage to native plants but is labor intensive and too expensive except in small patches (Pasiecznik 2016).
In addition to the conventional control methods, a classical biological control program for Tamarix spp. was initiated in the 1960s in California and continued as a major US Department of Agriculture–Agricultural Research Service program (USDA-ARS) in the 1980s and early 1990s with overseas explorations in France, Israel, Turkmenistan, China and Kazakhstan (DeLoach et al. 2000, 2014). Surveys of Tamarix natural enemies in the weed’s native range resulted in the enumeration of over 100 associated herbivores (Carruthers et al. 2008) and various populations of what was considered the leaf beetle Diorhabda elongata (Brullé) were collected in Eurasia and North Africa between 1992 and 2002. More recently, however, a taxonomic revision by Tracy and Robins (2009) organized a complex of five fully diagnosable species of tamarisk beetles. Although the four closely related Diorhabda species used in the North American tamarisk biological control program (Table 1) were separable using molecular methods, hybrids could be formed by making interspecific crosses between these four species, particularly between the Mediterranean tamarisk beetle and the subtropical tamarisk beetle (Bean et al. 2013). Based on this revision, it is now understood that Diorhabda carinulata (Desbrochers) was imported to the USDA containment facilities in Temple, Texas and Albany, California for host specificity testing (DeLoach et al. 2003; Lewis et al. 2003a, b; Milbrath and DeLoach 2006; Herr et al. 2014). After seven years of study, D. carinulata was determined to be sufficiently host-specific for introduction into the US in July 1999 (Carruthers et al. 2008). Initial releases were made in 2001 in the states of California, Colorado, Nevada, Texas, Utah, and Wyoming (DeLoach et al. 2014). Although most of the beetle releases did not result in establishment (DeLoach et al. 2004), at some sites the beetle overwintered, dispersed, and the resulting defoliation of extensive Tamarix stands in select areas has been reported (Carruthers et al. 2008). By 2014 this species had controlled a total of 5335 stream km of Tamarix in Nevada, Utah, Colorado, north western New Mexico, and northern Arizona (DeLoach et al. 2014). Three other Diorhabda species have also been introduced into the USA: D. sublineata (Lucas), D. carinata (Faldermann) and D. elongata. D. elongata was released in California in 2003 and all three were released in west Texas since 2006. Releases of the Diorhabda spp. in California and Texas resulted in lower levels of control than D. carinulata, but included ca. 40% of the Tamarix-infested riparian areas in Texas and defoliation was observed over large areas for 2–4 consecutive years (Knutson et al. 2014). Diorhabda spp. adults and larvae feed on the foliage and sometimes also on the epidermis of young shoots of Tamarix. The larvae pupate under litter on the ground and the adults overwinter there (Lewis et al. 2003a, b). Day length appears to be the strongest factor inducing overwintering diapause for D. carinulata, and likely the other three sp. released in the USA (Bean et al. 2012; Dalin et al. 2010). The successful population buildup of D. carinulata and resulting damage to Tamarix in North America is influenced by adaptations to critical day lengths, with adults seeking overwintering sites when days are lower than 14 to 15 h for given latitudes (Bean et al. 2012).
In Argentina, attempts to control Tamarix, have focused on physical, mechanical and chemical methods (Sosa et al. 2006; Natale et al. 2014). In 2006, a Tamarix eradication program was implemented at the Llancanelo Lake Provincial Natural Reserve in Mendoza (Sosa et al. 2006). Small plants and shrubs of Tamarix were extracted by hand or with hand tools in an area of ca. 12 ha around the lake edge (Sosa et al. 2006). At the same lake, the municipality of Malargüe, Provincial Park Administration and local communities implemented a monitoring and control program for Tamarix that involves felling the trees with chainsaws (http://losandes.com.ar/article/view?slug=erradicaran-los-tamarindos-en-llancanelo). In addition, a pilot research program for Tamarix spp. is being implemented as part of the NSIAS in two natural protected areas of high conservation value in the Cuyo region (FAO GEF 2017). The objective of the research program is to implement a management strategy for Tamarix and restoration of biodiversity and ecosystem services in 180 ha (90 ha in the Llancanelo lake area and 90 ha in the area of the Guanacache, Desaguadero and Del Bebedero lakes) (FAO GEF 2017).
Potential for classical biological control of Tamarix in Argentina
A standard practice of a CBC programs involves documentation of herbivores attacking the target weed in its adventive range to determine if natural enemies must be introduced from the native range of the weed. A few generalist insect herbivores are known to be associated with Tamarix in Argentina: Ceroplastes formicarius Hempel, Ceroplastes sp., Coccus hesperidum (L.) (Hemiptera: Coccidae), Automeris aspersa (Felder), A. melanops (Walker) (Lepidoptera: Saturniidae), Criptoblabes gnidiela (Milliere) (Lepidoptera; Pyralidae), Oiketicus kirbyi Guilding, O. platensis Berg (Lepidoptera: Psychidae), Tolype pauperata (Burmeister) (Lepidoptera: Lasiocampidae), Bostrichopsis uncinata Germ. (Coleoptera: Bostrichidae), and Praxithea deroudei (Chabrillac) (Coleoptera: Cerambicidae) (Cordo et al. 2004; Pastrana 2004). In addition, the exotic leafhopper Opsius stactogalus Fieber (Hemiptera: Cicadellidae), which is specific to the genus Tamarix, has been found to be common and abundant in Argentina, but it is not reported to cause any significant damage to Tamarix (Virla et al. 2010). This leafhopper, which has also been recorded in the USA, can cause significant damage to Tamarix plants in a cage setting (Louden 2010). However, the impact of this species in the field for controlling the growth and spread of naturalized tamarisk is generally thought to be insignificant (DeLoach et al. 2004). Considering that insect species associated to Tamarix in Argentina do not suppress plant fitness, the introduction of additional host-specific insects or pathogens may be the only economically feasible method to control the density and spread of Tamarix spp. on a large scale.
However, biological control is not a panacea and risks of introducing an exotic species must be considered. Risk of damage to non-target plants is highest with plants that are most closely related to the target species (Pemberton 2000). The relative taxonomic isolation of Tamarix is an important aspect in evaluating the safety of Diorhabda in Argentina. Host specificity studies conducted in the USA determine that the Diorhabda beetles host range was restricted to plant species of Tamaricaceae (Tamarix and the old World genus Myricaria) and Frankeniaceae (Frankenia) (DeLoach et al. 2003; Milbrath and DeLoach 2006). No Diorhabda larvae completed development on any of the other 54 species of Caryophyllales tested (e.g. Amaranthaceae, Droseraceae, Plumbaginaceae, Polygonaceae, Portulacaceae, Simmondsiaceae). Other plant species unrelated to Tamarix, but that shared its habitat (e.g. Asteraceae, Fabaceae, Salicaceae), and commercial crop plants (e.g. Vitis vinifera L., Helianthus annuus L., Juglans regia L., Lactuca sativa L., Cucumis sativus L., Prunus americana Marshall, Prunus dulcis (Miller) D.A. Webb, Lycopersicon esculentum and Triticum aestivum L.) were also tested, with negative results (DeLoach et al. 2003; Milbrath and DeLoach. 2006).
Open-field studies indicated that Diorhabda beetles could not thrive on Frankenia, although some adult and larval damage was observed on F. salina when Diorhabda beetle densities were extremely high, and all of the surrounding Tamarix had been totally defoliated (Dudley and Kazmer 2005). An open review process involving scientific representatives from various stakeholder organizations (state, county, university, and environmental groups) supported the redistribution of Diorhabda beetles in California owing to its impact on Tamarix and its negligible non-target effects (Herr et al. 2014).
Shipments of Diorhabda beetles established in North America could be obtained through international cooperation with the USA (e.g. USDA-ARS) to start a colony on potted plants of Tamarix at the National Institute of Agricultural Technology (Instituto Nacional de Tecnología Agropecuaria, INTA) containment facility, in Castelar, Buenos Aires, Argentina. Ten species of Frankenia occur in southern South America, seven of which occur in Argentina (Zuloaga and Morrone 2009) and constitute the only plants in the continent that are somewhat related to Tamarix. A test plant list for Argentina should include all the Frankenia species present in the country. In addition, upon request of Argentine regulatory authorities, certain species within non-core Caryophyllales families (e.g. Plumbaginaceae and Polygonaceae) could also be included in the specificity tests.
Suitability of Diorhabda species to Tamarix invasion in Argentina
To investigate Argentina’s climate suitability for colonization by Diorhabda species, we modeled the potential distribution and resulting overlap of D. carinulata, D. sublineata, D. elongata, and D. carinata with their host T. ramosissima using the software MaxEnt, version 3.3.3 k (Phillips et al. 2006, found at http://biodiversityinformatics.amnh.org/open_source/maxent/). Presence data (GPS coordinates) for the Diorhabda spp. were extracted from Tracy and Robbins (2009), Nagler et al. (2014), and Sánchez-Peña et al. (2016) (Table 2). Occurrence data for T. ramosissima in Argentina was acquired from Natale et al. (2008) along with data from the Global Biodiversity Information Facility’s website (http://www.GBIF.org).
Altitude and 19 bioclimatic variables were downloaded in 2.5 arc-minute resolution raster format from WorldClim’s online database (Hijmans et al. 2005). These data are interpolated from weather station data, and are derivations of temperature and precipitation data considered to be biologically relevant (Hijmans et al. 2005). Default MaxEnt settings were used (Rinnhofer et al. 2012; Mukherjee et al. 2012), with 10,000 pseudo-absence points randomly selected from the model background. Models were run ten times, with 70% of the dataset randomly selected for training to build the models and the remaining 30% used for testing (Mukherjee et al. 2011). The continuous outputs were converted to the binary categories of suitable or unsuitable habitat to simplify comparison of the two species’ ranges. The maximum sum of sensitivity and specificity of training data was used as a threshold to convert output of continuous to binary values (Liu et al. 2013). ArcMap’s Raster Calculator tool was used to calculate the predicted range overlap of the two binary suitability outputs (ESRI 2014).
The resulting models were tested using one-tailed binomial t-tests to determine if modeled predictions were significantly better than random models with the same fractional predicted area. The omission rate, or percentage of test points that fell outside of the binary predicted range, was also tested within MaxEnt. Additionally, ENMTools’ Range Overlap tool was used to compare the beetle’s individual habitat suitability models with that of T. ramossissima (Warren et al. 2008, 2010).
Each species’ model predictions proved to be significantly better than random, with all 50 replicates of binary predictions having p-values < 0.01. All area under the curve (AUC) values were > 0.9 and thus considered “good” (Pearson et al. 2006) (Table 2). Test omission rates were similarly small, indicating limited error in the models. The subtropical tamarisk beetle, D. sublineata, had a markedly higher suitability value across Argentina as compared to its congeners (Table 2; Fig. 3). Range overlap between T. ramosissima and D. sublineata is estimated to be 44.2%, (Fig. 4) while T. ramosissima and the other three beetles had a near 0% overlap. The model results for D. carinulata, arguably the most important biological control agent of Tamarix in the USA, failed to identify any areas in Argentina deemed suitable habitat for the beetle (Fig. 5).
These models comparing environmental suitability indicate that climatic factors may markedly influence the colonization of T. ramosissima by the Diorhabda beetles if released in Argentina. Models predict two regions—one on the eastern coast and one in the northwest region of Argentina—that are predicted to be more suitable for colonization by D. sublineata (Fig. 3). Data also indicate, however, that the remaining natural enemies are poorly adapted to conditions in Argentina generally. Graphical results are presented for D. carinulata (Fig. 5) but similar output was also observed for D. elongata and D. carinata. These findings are not unlike the realized allopatric distributions of Diorhabda spp. in North America, with D. carinulata occurring primarily within the Great Basin and now dispersing into southern California while D. sublineata is established in southern Texas and is dispersing south into Mexico (Estrada-Muñoz and Sánchez-Peña 2014; Sánchez-Peña et al. 2016).
It is clear that environmental factors will contribute to establishment and spread of the Diorhabda beetles in Argentina, but day length requirement is also a strong driver determining the suitability of beetles as biological control agents of Tamarix. As stated previously, populations of Diorhabda beetles in the USA respond to critical day lengths ≤ 14 h by switching from reproductive to overwintering strategies (Bean et al. 2012). In Cordoba, Argentina, a city near large stands of T. ramosissima, the longest day of the year experiences just 14 h of daylight. This indicates that current D. carinulata ecotypes present in the USA are poorly adapted to day length regimes in the weed’s invaded range in Argentina, although in the USA D. carinulata is adapting to shorter critical day length over time (Bean et al. 2012). In contrast, D. sublineata appears to be better adapted to short critical day lengths in the USA and may be the best candidate for initial consideration for Argentina (Tracy and Robbins 2009).
Discussion
The taxonomic isolation of Tamarix spp. in Argentina, their widespread distribution, negative impact to natural areas and lack of impact from existing natural enemies all indicate that Tamarix is an ideal candidate for classical biological control in Argentina.
Piggybacking on the biocontrol program undertaken in the USA constitutes an opportunity for Argentina to reduce costs associated with the exploratory surveys, risk assessments and rearing procedures to implement biological control of Tamarix spp. in the country. Considering the host range tests performed in the USA and the taxonomic isolation of Tamarix spp. in Argentina, a minimal amount of additional host specificity testing would have to be conducted in Argentina to evaluate if Diorhabda beetles could be safely used as biological control agents against Tamarix spp. in Argentina.
Classical biological control implementation is often considered only after conventional control methods are deemed unsustainable or have failed to reduce weed population levels (Olckers 2004). However, high rates of success can be achieved by targeting weeds during the early stages of invasion (Olckers 2004). Tamarix spp. are widespread in Argentina, but its potential for invasion is probably at an early stage. Tamarix spp. have the potential to invade more than three quarters of the arid and semi-arid areas of the country (around 1.4 million km2), constituting one of the main threats to biodiversity conservation (Natale et al. 2013). This implies that the problem could be attacked before it becomes even more widespread. Manual and herbicidal control can be effective in some areas, such as public parks and protected areas, but these approaches are not feasible at a landscape scale. In this context, it is proposed that integration of biological control with existing short-term control measures will reduce Tamarix abundance and spread. The implementation of a CBC program for Tamarix in Argentina is expected to contribute to the restoration of biodiversity and ecosystem services in protected areas of high conservation value and to the conservation of water resources in sensitive arid and semi-arid regions of Argentina.
References
APN-SIB (2016) Administración de parques nacionales-sistema de información de biodiversidad. Available via DIALOG. http://www.sib.gov.ar. Accessed on 11 May 2015
Bean DW, Dudley TL, Keller JC (2007) Seasonal timing of diapause limits the effective range of Diorhabda elongata deserticola (Coleoptera: Chrysomelidae) as a biological control agent for tamarisk (Tamarix spp.). Environ Entomol 36:15–25
Bean DW, Dalin P, Dudley TL (2012) Evolution of critical day length for diapause induction enables range expansion of Diorhabda carinulata, a biological control agent against tamarisk (Tamarix spp.). Evol Appl 5:511–523
Bean DW, Kazmer DJ, Gardner K, Thompson DC, Reynolds B, Keller JC, Gaskin JF (2013) Molecular genetic and hybridization studies of Diorhabda spp. released for biological control of Tamarix. Invasive Plant Sci Manag 6:1–15
Beauchamp VB, Stromberg JC, Stutz JC (2005) Interactions between Tamarix ramosissima (saltcedar), Populus fremontii (cottonwood), and mycorrhizal fungi: effects on seedling growth and plant species coexistence. Plant Soil 275:221–231
BirdLife International (2017) Species factsheet: Buteogallus coronatus. Available via DIALOG. http://datazone.birdlife.org/species/factsheet/crowned-solitary-eagle-buteogallus-coronatus. Accessed on 25 Jan 2017
Carruthers RI, Herr JC, DeLoach CJ (2006) An overview of the biological control of saltcedar. In: Hoddle MS, Johnson MW (eds), Proceedings of the California conference on biological control. University of California, Riverside, California, USA, pp 128–134
Carruthers RI, DeLoach CJ, Herr JC, Anderson GL, Knutson AE (2008) Saltcedar areawide pest management in the western USA. In: Opender K, Cuperus G, Elliott N (eds) Areawide pest management theory and implementation. CAB International, Wallingford, pp 271–299
Cooper DJ, Andersen DC, Chimner RA (2003) Multiple pathways for woody plant establishment on floodplains at local to regional scales. J Ecol 91:182–196
Cordo HA, Logarzo G, Braun K, di Iorio OR (2004) Catálogo de insectos fitófagos de la Argentina y sus plantas asociadas. Sociedad entomológica argentina, Buenos Aires
Crins WL (1989) The Tamaricaceae in the southeastern United States. J Arnold Arbor 70:403–425
Dalin P, Bean DW, Dudley TL, Carney VA, Eberts D, Gardner KT, Hebertson E, Jones EN, Kazmer DJ, Michels GJ Jr (2010) Seasonal adaptations to day length in ecotypes of Diorhabda spp. (Coleoptera: Chrysomelidae) inform selection of agents against saltcedars (Tamarix spp.). Environ Entomol 39:1666–1675
DeLoach CJ, Carruthers RI, Lovich JE, Dudley TL, Smith SD (2000) Ecological interactions in the biological control of Saltcedar (Tamarix spp.) in the United States: toward a new understanding. In: Spencer NR (ed) Proceedings of the X international symposium on biological control weeds. Montana State University, Bozeman, USA, pp 819–874
DeLoach CJ, Lewis PA, Herr JC, Carruthers RI, Tracy JL, Johnson J (2003) Host specificity of the leaf beetle, Diorhabda elongata deserticola (Coleoptera: Chrysomelidae) from Asia, a biological control agent for saltcedars (Tamarix: Tamaricaceae) in the western United States. Biol Control 27:117–147
DeLoach CJ, Carruthers RI, Dudley TL, Eberts D, Kazmer DJ, Knutson AE, Bean DW, Knight J, Lewis PA, Milbrath LR, Tracy JL, Tomic-Carruthers N, Herr JC, Abbott G, Prestwich S, Harruff G, Everitt JH, Thompson DC, Mityaev I, Jashenko R, Li B, Sobhian R, Kirk A, Robbins TO, Delfosse ES (2004) First results for control of saltcedar (Tamarix spp.) in the open field in the western United States. In: Cullen JM, Briese DT, Kriticos DJ, Lonsdale WM, Morin L, Scott JK (eds) Proceedings of the XI international symposium on biological control of weeds. CSIRO entomology, Canberra, Australia, pp 505–513
DeLoach CJ, Carruthers R, Knutson A, Bean D, Tracy J, Moran PJ, Herr JC, Gaskin JF, Dudley TL, Ritzi CM, Michaels GJ (2014) Biological control of saltcedar (Tamarix: Tamaricales) in the western USA: control and environmental effects progress since 2010. Tamarix workshop. In: Impson FAC, Kleinjan CA, Hoffmann JH (eds) Proceedings of the XIV international symposium on biological control of weeds. University of Cape Town, Kruger National Park, South Africa, pp 191–192
Drus GM, Dudley TL, Brooks ML, Matchett JR (2012) The effect of leaf beetle herbivory on the fire behaviour of tamarisk (Tamarix ramosissima Lebed.). Int J Wildland Fire 22:446–458
Dudley TL, Bean DW (2012) Tamarisk biocontrol, endangered species risk and resolution of conflict through riparian restoration. BioControl 57:331–347
Dudley TL, DeLoach CJ (2004) Saltcedar (Tamarix spp.), endangered species and biological weed control—can they mix? Weed Technol 18:1542–1551
Dudley TL, Kazmer DJ (2005) Field assessment of the risk posed by Diorhabda elongata, a biocontrol agent for control of saltcedar (Tamarix spp.), to a nontarget plant, Frankenia salina. Biol Control 35:265–275
Egan TB (1997) Afton Canyon riparian restoration project: fourth year status report. USDI Bureau of Land Management, Barstow, California
Environmental Systems Research Institute (ESRI) (2014) ArcGIS desktop: release 10.2. Environmental Systems Research Institute, Redlands, CA
Estrada-Muñoz GA, Sánchez-Peña SR (2014) Imidacloprid drench on Athel trees (Tamarix aphylla): effect on foliage consumption and knock-down of Diorhabda sublineata at Chihuahua, Mexico. Southwest Entomol 39:439–450
FAO GEF (2017) Strengthening governance for biodiversity protection by formulating and implementing the national strategy on invasive alien species. Available via DIALOG. https://www.thegef.org/sites/default/files/project_documents/11-24-14_-_ProDoc_0.pdf. Accessed on 10 Dec 2016
Gaskin JF, Kazmer DJ (2009) Introgression between invasive saltcedars (Tamarix chinensis and T. ramosissima) in the USA. Biol Invasions 11:1121–1130
Gaskin JF, Ghahremani-nejad F, Zhang D-Y, Londo JP (2004) A systematic overview of Frankeniaceae and Tamaricaceae from nuclear rDNA and plastid sequence data. Ann Missouri Bot Gard 91:401–409
GISP (2007) Global invasive species programme. Invasive species and poverty: exploring the links. Cape Town, South Africa. Available via DIALOG: http://www.gisp.org. Accessed on 15 Aug 2016
Harley KL, Forno IW (1992) Biological control of weeds. A handbook for practitioners and students. Inkata Press, Melbourne
Herr JC, Herrera-Reddy AM, Carruthers RI (2014) Field testing Diorhabda elongata (Coleoptera: Chrysomelidae) from Crete, Greece, to assess potential impact on nontarget native California plants in the genus Frankenia. Environ Entomol 43:642–653
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hultine KR, Dudley TL, Koepke DF, Bean DW, Glenn EP, Lambert AM (2015) Patterns of herbivory-induced mortality of a dominant non-native tree/shrub (Tamarix spp.) in a southwestern US watershed. Biol Invasions 17:1729–1742
Hurrell JA, Bazzano D, Delucchi G (2004) Arbustos 2 nativos y exóticos. Biota rioplatense. LOLA, Buenos Aires
INBIAR-UNS (2016) Invasiones biológicas en Argentina-universidad Nacional del sur. Available via DIALOG. http://www.inbiar.uns.edu.ar. Accessed on 10 Jan 2017
Karlin UO, Goirán SB, Karlin M (2010) Los usos de las principales plantas de las Salinas Grandes. Universidad Nacional de Córdoba-Facultad de Ciencias Agrarias, Córdoba
Knutson K, Tracy J, Michels J, Jones E, Muegge M, Ritzi C, DeLoach J (2014) Establishment, dispersal and impact of three species of Diorhabda leaf beetles released for the biological control of saltcedars, Tamarix species, in Texas. In: Impson FAC, Kleinjan CA, Hoffmann JH (eds) Proceedings of the XIV international symposium on biological control of weds. University of Cape Town, Kruger National Park, South Africa, pp 96
Lewis PA, DeLoach CJ, Knutson AE, Tracy JL, Robbins TO (2003a) Biology of Diorhabda elongata deserticola (Coleoptera: Chrysomelidae), an Asian leaf beetle for biological control of saltcedars (Tamarix spp.) in the United States. Biol Control 27:101–116
Lewis PA, DeLoach CJ, Herr JC, Dudley TL, Carruthers RI (2003b) Assessment of risk to native Frankenia shrubs from an Asian leaf beetle, Diorhabda elongata deserticola (Coleoptera: Chrysomelidae), introduced for biological control of saltcedars (Tamarix spp.) in the western United States. Biol Control 27:148–166
Liu C, White M, Newell G (2013) Selecting thresholds for the prediction of species occurrence with presence-only data. J Biogeogr 40:778–789
Louden NP (2010) Asymmetric interspecific competition between specialist herbivores that feed on tamarisk in western Colorado. M.Sc. Thesis. Utah State University
Maceda JJ (2007) Biología y conservación del Águila Coronada (Harpyhaliaetus coronatus) en Argentina. Hornero 22:159–171
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Milbrath LR, DeLoach CJ (2006) Host specificity of different populations of the leaf beetle Diorhabda elongata (Coleoptera: Chrysomelidae), a biological control agent of saltcedar (Tamarix spp.). Biol Control 36:32–48
Mukherjee A, Christman MC, Overholt WA, Cuda JP (2011) Prioritizing areas in the native range of hygrophila for surveys to collect biological control agents. Biol Control 56:254–262
Mukherjee A, Williams DA, Wheeler GS, Cuda JP, Pal S, Overholt WA (2012) Brazilian peppertree (Schinus terebinthifolius) in Florida and South America: evidence of a possible niche shift driven by hybridization. Biol Invasions 14:1415–1430
Nagler PL, Pearlstein S, Glenn EP, Brown TB, Bateman HL, Bean DW, Hultine KR (2014) Rapid dispersal of saltcedar (Tamarix spp.) biocontrol beetles (Diorhabda carinulata) on a desert river detected by phenocams, MODIS imagery and ground observations. Rem Sens Environ 140:206–219
Natale E, Gaskin J, Zalba SM, Ceballos M, Reinoso H (2008) Especies del género Tamarix (Tamarisco) invadiendo ambientes naturales y seminaturales en Argentina. Bol Soc Argent Bot 43:137–145
Natale E, Zalba SM, Oggero A, Reinoso H (2010) Establishment of Tamarix ramosissima under different conditions of salinity and water availability: implications for its management as an invasive species. J Arid Environ 74:1399–1407
Natale E, Zalba SM, Reinoso H (2012) Assessing invasion process through pathway and vector analysis: case of Saltcedar (Tamarix spp.). Manag Biol Invasion 3:37–44
Natale E, Zalba SM, Reinoso H (2013) Presence–absence versus invasive status data for modelling potential distribution of invasive plants: saltcedar in Argentina. Ecoscience 20:161–171
Natale E, Oggero A, Marini D, Reinoso H (2014) Restauración de bosque nativo en un área invadida por tamariscos Tamarix ramossisima en el sur de la provincia de Córdoba, Argentina. Ecosistemas 23:130–136
Olckers T (2004) Targeting emerging weeds for biological control in South Africa: the benefits of halting the spread of alien plants at an early stage of their invasion. S Afr J Sci 100:64–68
Pasiecznik N (2016) Tamarix ramosissima. In: Invasive species compendium. Available via DIALOG. https://www.cabi.org/isc/datasheet/52503. Accessed on 1 Dec 2016
Pastrana JA (2004) Los lepidópteros argentinos. Sus plantas hospedadoras y otros sustratos alimenticios. Sociedad entomológica argentina, Buenos Aires
Pattison RR, D’Antonio CM, Dudley TL, Allander KK, Rice B (2011) Early impacts of biological control on canopy cover and water use of the invasive saltcedar tree (Tamarix spp.) in western Nevada, USA. Oecologia 165:605–616
Pearson RG, Thuiller W, Araujo MB, Martinez-Meyer E, Brotons L, McClean C, Miles L, Segurado P, Dawson TP, Lees DC (2006) Model-based uncertainty in species range prediction. J Biogeogr 33:1704–1711
Pemberton RW (2000) Predictable risk to native plants in weed biological control. Oecologia 125:489–494
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259
Richardson DM, Rejmánek M (2011) Trees and shrubs as invasive alien species – a global review. Divers Distrib 17:788–809
Richardson DM, Hui C, Nuñez MA, Pauchard A (2014) Tree invasions: patterns, processes, challenges and opportunities. Biol Invasions 16:473–481
Rinnhofer LJ, Roura-Pascual N, Arthofer W, Dejaco T, Thaler-Knoflach B, Wachter GA, Christian E, Steiner FM, Schlick-Steiner BC (2012) Iterative species distribution modelling and ground validation in endemism research: an alpine jumping bristletail example. Biodivers Conserv 21:2845–2863
Sánchez-Peña SR, Morales-Reyes C, Herrera-Aguayo F, Torres-Acosta I, Camacho-Ponce D, Gonzalez-Gallegos E, Ritzi C, Sirotnak J, Briggs M (2016) Distribution of the subtropical tamarisk beetle, Diorhabda sublineata (Lucas, 1849) (Coleoptera: Chrysomelidae), in Mexico. Pan-Pac Entomol 92:56–62
Shafroth PB, Cleverly J, Dudley TL, Stuart J, van Riper C, Weeks EP, Stuart JN (2005) Saltcedar removal, water salvage and wildlife habitat restoration along rivers in the southwestern U.S. Environ Manag 35:231–246
Simberloff D, Martin J-L, Genovesi P, Maris V, Wardle DA, Aronson J, Courchamp F, Galil B, García-Berthou E, Pascal M, Pyšek P, Sousa R, Tabacchi E, Vilà M (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66
Sogge MK, Sferra SJ, Paxton EH (2008) Tamarix as habitat for birds: implications for riparian restoration in the southwestern United States. Restor Ecol 16:146–154
Sosa H, Cruces E, Munafó L, Tamarit R, Martín S, Aranda L, Cornetti E (2006) Prueba de erradicación manual de parches herbáceos y arbustivos de tamarindo (Tamarix sp.) en sitio Ramsar laguna Llancanelo Malargüe, Mendoza Argentina. II jornadas nacionales de flora nativa. III encuentro de cactáceas. Comisión de flora nativa, Mendoza, Argentina, pp 28–31
Stevens PF (2001). Angiosperm phylogeny website. Version 9, June 2008. Available via DIALOG http://www.mobot.org/MOBOT/research/APweb/. Accessed on 5 Nov 2016
Tracy JL, Robbins TO (2009) Taxonomic revision and biogeography of the Tamarix-feeding Diorhabda elongata (Brullé, 1832) species group (Coleoptera: Chrysomelidae: Galerucinae: Galerucini) and analysis of their potential in biological control of Tamarisk. Zootaxa 2101:1–152
van Driesche RG, Carruthers RI, Center T, Hoddle MS, Hough-Goldstein J, Morin L, Smith L, Wagner DL, Blossey B, Brancatini V, Casagrande R, Causton CE, Coetzee JA, Cuda J, Ding J, Fowler SV, Frank JH, Fuester R, Goolsby J, Grodowitz M, Heard TA, Hill MP, Hoffmann JH, Hubert J, Julien M, Kairo MTK, Kenis M, Mason P, Medal J, Messing R, Miller R, Moore A, Neuenschwander P, Newman R, Norambuena H, Palmer WA, Pemberton R, Perez Panduro A, Pratt PD, Rayamajhi M, Salom S, Sands D, Schooler S, Schwarzländer M, Sheppard A, Shaw R, Tipping PW, van Klinken RD (2010) Classical biological control for the protection of natural ecosystems. Biol Control 54:2–33
Virla EG, Logarzo GA, Paradell SL (2010) Occurrence of the Tamarix leafhopper, Opsius stactogalus Fieber (Hemiptera: Cicadellidae), in Argentina. J Insect Sci 10:23
Warren DL, Glor RE, Turelli M (2008) Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62:2868–2883
Warren DL, Glor RE, Turelli M (2010) ENMTools: a toolbox for comparative studies of environmental niche models. Ecography 33:607–611
Whalen MA (1987) Systematics of Frankenia (Frankeniaceae) in North and South America Syst Bot. Monogr 17:1–93
Zavaleta E (2000) Valuing ecosystem services lost to Tamarix invasion in the United States. In: Mooney HA, Hobbs RJ (eds) Invasive species in a changing world. Island Press, Washington, DC, pp 261–300
Zuloaga F, Morrone O (2009) Flora del conosur. Catálogo de las plantas vasculares. Instituto de botánica “Darwinion”, Buenos Aires. Avialable via DIALOG. http://www2.darwin.edu.ar/Proyectos/FloraArgentina/fa.htm. Accessed on Dec 2015
Acknowledgements
We wish to thank Jack DeLoach and Ray Carruthers for their advice during preliminary stages of this review. This project was funded by the Foundation for the Study of Invasive Species and the United States Department of Agriculture, Agricultural Research Service.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: S. Raghu.
Rights and permissions
About this article
Cite this article
Mc Kay, F., Logarzo, G., Natale, E. et al. Feasibility assessment for the classical biological control of Tamarix in Argentina. BioControl 63, 169–184 (2018). https://doi.org/10.1007/s10526-017-9855-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-017-9855-3