Abstract
Streptomyces is a genus known for its ability to protect plants against many pathogens and various strains of this bacteria have been used as biological control agents. In this study, the efficacy of Streptomyces philanthi RM-1-138, S. philanthi RL-1-178, and Streptomyce mycarofaciens SS-2-243 to control various strains of Botrytis cinerea was evaluated both in vitro and in vivo. In vitro studies using confrontation tests on PDA plates indicated that the three strains of Streptomyces spp. inhibited the growth of 41 strains of B. cinerea. Volatile compounds produced by Streptomyces spp. had an influence on the growth of ten strains of B. cinerea while its culture filtrate at low concentration (diluted at 10−3) showed a complete inhibition (100%) of spore germination of B. cinerea strain BC1. A significant protection efficacy of tomato against B. cinerea was observed on both whole plant test (57.4%) and detached leaf test (60.1%) with S. philanti RM-1-138. Moreover, this antagonistic strain had a preventive and a curative effect. These results indicated that S. philanthi RM-1-138 may have the potential to control gray mold caused by B. cinerea on tomato but further work is required to enhance its efficacy and its survival in planta.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gray mold disease caused by the fungus Botrytis cinerea Pers. [teleomorph Botryotinia fuckeliana (de Bary) Whetzel] is an economically important disease on numerous vegetable including tomato (Jarvis 1980; Helbig 2002; Elad et al. 2015). Botrytis cinerea can infect the plants either by direct penetration or through wounds caused by cultivation practices. Infection is promoted by high humidity, free moisture on the plant surface, and low temperatures (Lee et al. 2006). In general, disease management strategy largely relies on the use of chemical fungicides that are generally considered harmful to the environment and can result in fungicide resistance as previously reported (Locke and Fletcher 1988; Yourman and Jeffers 1999; Chung et al. 2006). The development of efficient antifungal microbial agents could be an alternative method to control the disease.
Species of Streptomyces are potential biocontrol agents since they are ubiquitous in the environment and many of them produce secondary metabolites such as enzyme inhibitors and antibiotics with diverse biological activities, including the ability to inhibit plant pathogenic fungi (Omura 1992; Lange and Sanchez 1996; Vaz Jauri et al. 2016). Several species of Streptomyces have been isolated and used to control plant pathogens on various crops, such as Pyricularia oryzae and Rhizoctonia solani on rice (Prabavathy et al. 2006), R. solani and Sclerotium rolfsii on sugar beet (Sadeghi et al. 2006; Errakhi et al. 2007) and Sclerotinia sclerotiorum on sunflower (Baniasadi et al. 2009). In particular, strain RM-1-138 and RL-1-178 of S. philanthi and strain SS-2-243 of S. mycarofaciens, isolated from the rhizosphere of chili pepper in southern Thailand (Boukaew et al. 2011), have shown a good efficacy to control S. rolfsii, Ralstonia solanacearum on chili (Boukaew et al. 2011), and Rhizoctonia solani on rice (Boukaew et al. 2013, 2014a, b). However, few studies explored the effect of Streptomyces sp. on B. cinerea. For instance, the strain A01 of Streptomyces lydicus produces natamycin and chitinase that have the potential to control gray mold caused by B. cinerea (Wu et al. 2013).
In all the studies cited above, the effect of the biocontrol agent Streptomyces spp. was tested against a single strain of the plant pathogen considered. However, some studies highlight differences in the sensitivity of various isolates of plant pathogens to biocontrol agents, with the existence of less sensitive isolates in natural populations of plant pathogens and even the capacity of plant pathogens to adapt to biocontrol agents (Bardin et al. 2015). Diversity of sensitivity of plant pathogens to biocontrol agents may result in a reduction of efficacy of the biocontrol agent in the field and if selection pressure would increase, i.e., intensive use of biocontrol agents by growers, we can expect a selection of the less sensitive isolates and consequently a reduced efficacy of biocontrol agents against plant pathogens in the field.
The first objective of the present study was to evaluate the ability of three strains of Streptomyces spp. to inhibit the growth of B. cinerea in vitro and on tomato plants. The three tested strains, RM-1-138 and RL-1-178 of Streptomyces philanthi and SS-2-243 of S. mycarofaciens, are known for their antifungal effect on various plant pathogens (Boukaew et al. 2011, 2013, 2014a, b), but they have never been tested against B. cinerea. The second objective of the study was to assess the possible variability in susceptibility of various strains of B. cinerea to these antagonistic strains. To this end, 41 strains of B. cinerea differing in their date of isolation, geographic and host or substrate of origin were used.
Materials and methods
Microorganisms and inoculum preparation
Strains RM-1-138 and RL-1-178 of S. philanthi and strain SS-2-243 of S. mycarofaciens were isolated in a previous study from the rhizosphere of chili pepper in southern Thailand (Boukaew et al. 2011). Spores of Streptomyces were collected in 10 ml water from ten-day-old culture on glucose yeast-malt agar at 30 °C and counted using a hematocytometer. Inoculum was prepared by dilution in distilled sterilized water to achieve the required concentration.
Forty-one strains of Botrytis cinerea differing in their date of isolation, geographic and plant hosts or substrate of origin were used in this study. All isolates were single-spored and conserved at −20 °C before use. In all tests, the B. cinerea strain BC1, known to be very aggressive on tomato (Decognet et al. 2009), was used. An inoculum of B. cinerea was prepared by cultivating the strain on potato dextrose agar (PDA) plates under cool white fluorescent light (14 h photoperiod at 65 µmol m−2 s−1) in a growth chamber at 21 °C. Spores were collected in 5 ml water from 14-day-old culture and counted using a hematocytometer. Inoculum was prepared by dilution in distilled sterilized water to achieve the required concentration.
Dual culture
The three strains of Streptomyces spp. were evaluated for their antagonistic properties against 41 strains of B. cinerea using a dual culture technique (Islam et al. 2009). For each strain of Streptomyces spp., a streak of spore suspension at 107 spores ml−1 was deposited on one side of a PDA medium in Petri dishes. In previous study, Boukaew et al. (2011) have shown that the antagonistic activity of the three strains of Streptomyces by dual culture on agar was observed after seven days of incubation at 28 °C. For RM-1-138 the optimal activity of antifungal metabolites was observed for culture filtrate obtained after 9–11 days of incubation at 28 °C (Boukaew and Prasertsan 2014b). Plates were then incubated in a growth chamber for ten days at 21 °C in the dark.
A 5-mm-diameter mycelial plug, excised from a three-day-old B. cinerea colony, was transferred to the center of each plate. As a control, a mycelial plug of B. cinerea was placed on a PDA plate without any Streptomyces strain. The dual culture plates were further incubated in a growth chamber (21 °C, dark) for two days, after which the radial mycelial growth of B. cinerea was measured and compared to that of the control. Three replicates were conducted for each Streptomyces–B. cinerea strain combination. The colony size in each treatment was recorded and the percentage inhibition of hyphal growth was calculated.
Volatile effect
Streptomyces philanthi RM-1-138 and RL-1-178, and S. mycarofaciens SS-2-243 were evaluated for the effect of their volatile substances on the mycelial development of ten strains of B. cinerea. Strains of B. cinerea tested were selected based on their susceptibility to Streptomyces spp. in the dual culture assay: the five most sensitive strains and the five less sensitive strains to Streptomyces spp. were tested. For each strain of Streptomyces spp., a streak of a spore suspension at 107 spores ml−1 was deposited on PDA medium in a Petri dish. After a ten days incubation in a growth chamber (21 °C, dark) a Petri dish containing Streptomyces spp. was covered with another Petri dish containing PDA medium inoculated with a 5 mm-diameter mycelial plug of B. cinerea. The two plates were then sealed using double layers of parafilm to obtain a double-plate chamber (Raza et al. 2015). Double plates were incubated in a growth chamber at 21 °C (14 h day and 10 h night). After two days of incubation, the diameters of the colonies of B. cinerea were recorded, and the percentage inhibition of hyphal growth was calculated. For each treatment, three replicates were realized.
Culture filtrate effect
Streptomyces philanthi RM-1-138 and RL-1-178, and S. mycarofaciens SS-2-243 were each grown at 30 °C in a 250 ml flask containing 100 ml liquid GYM medium (glucose yeast-malt extract broth, pH adjusted at 7.0 before autoclaving) on a rotary shaker at 150 rpm. After three days of incubation, 5 ml aliquots of this culture was transferred into 100 ml fresh GYM medium, and incubated for ten days under the same conditions. The culture broth was centrifuged (8880×g for 20 min), then filtered through a 0.45 mm Millipore membrane to recover the culture filtrate.
Serial dilutions of the culture filtrate (1/10, 1/100 and 1/1000) were prepared in distilled sterile water. Ten µl of the different dilutions were then mixed with 2 µl of a spore suspension of the strain BC1 of B. cinerea dosed at 105 spores ml−1 and dropped on a glass-slide. Sterile Potato Dextrose Broth (PDB) was used instead of the culture filtrate as a positive control. The slides were further placed in Petri dishes with humid absorbent paper to maintain high relative humidity and incubated under cool white fluorescent light (14 h photoperiod at 65 µmol m−2 s−1) in a growth chamber at 21 °C for 24 h. Spores that germinate were counted under the microscope and the inhibition of spore germination (in percentage) was calculated.
Efficacy of Streptomyces spp. against B. cinerea on tomato
Seed of tomato cv. Monalbo (INRA Plant Pathology, Avignon, France) were sown in compost and transplanted after one week in an individual pot containing a horticulture compost mix (De Baat, Coevorden, The Netherlands). Plants were grown in a glasshouse for 7–8 weeks where they received a standard commercial nutrient solution once or twice a day, depending on needs. They had at least eight fully expended leaves when used.
Two types of test were performed to evaluate the efficacy of Streptomyces spp. to control B. cinerea on tomato plants: a detached leaf assay and a whole plant assay. Tests were first carried out with the three strains of Streptomyces spp. (RM-1-138, RL-1-178 and SS-2-243) against the strain BC1 of B. cinerea. Additional tests with RM-1-138 were realized with three strains of B. cinerea (BC1, C10 and CEN12-13) having different level of sensitivity to RM-1-138 in dual culture tests.
For the detached leaf assay, leaves were sprayed with a spore suspension of Streptomyces spp. at the concentration of 106 or 107 spores ml−1. When the leaves were dried (between 30 and 60 min after treatment), they were removed, and two leaflets were placed in a transparent plastic box with humid absorbent paper to maintain high relative humidity (close to 100%). Mycelial plugs (5-mm in diameter) of B. cinerea excised from the growing margin of a three-day-old PDA cultures were deposited onto the leaflets of tomato. For each treatment, six leaflets of tomato (three plastic boxes) were inoculated. Following inoculation, the detached leaves were incubated in a growth chamber in conditions conducive to disease development (21 °C, 14 h-photoperiod). Leaves removed from non-treated plants were inoculated as a control. The lesion area was determined with ImageJ software two days after inoculation. Three independent repetitions of the test were realized.
For the whole plant test, three leaves per plant were removed leaving 5–10 mm petiole stubs on the stems and each pruning wound was inoculated with 10 µl of a spore suspension of B. cinerea dosed at 106 spores ml−1, in order to place Botrytis in the most favorable conditions for infestation of the stem. Streptomyces spp. was then applied to pruning wounds with 10 µl of a spore suspension dosed at 106 or 107 spores ml−1, 5 min after the inoculation of B. cinerea, the time that the drop was absorbed by the petiole. The pathogen was inoculated alone as a control. All plants were incubated in a growth chamber with a photoperiod of 14 h and maintained at 21 °C with a relative humidity above 80% to favor disease development. The experiment was repeated three times independently, each with three replicates per treatment.
Lesion expansion on tomato stem was recorded daily for seven days after inoculation (DAI). To take into account the kinetics of disease development, we computed the area under the disease progress curves (AUDPC) as described by Decognet et al. (2009), as:
where Yj was the observed lesion length (in mm) at the jth observation time, n was the total number of observations, and I the interval between each observation (in days). These values were computed for individual pruning lesions for n = 5 observations at daily intervals during the period from the 3rd to the 7th day after inoculation. For both tests, a percentage of protection of tomato generated by the bacteria, compared to the control, was computed.
Preventive and curative effect of Streptomyces against B. cinerea on tomato plants
To test a potential preventive effect of the three strains of Streptomyces against B. cinerea on tomato plants, three leaves per plant were removed, leaving 5–10 mm petiole stubs on the stems. Each pruning wound was treated with 10 µl of a spore suspension at 107 spores ml−1 of a strain of Streptomyces sp. and then inoculated with 10 µl of a spore suspension at 106 spores ml−1 of the strain BC1 of B. cinerea. Inoculation of BC1 was realized 0, 2, 4 or 6 h after treatment with the selected strain of Streptomyces.
To test a potential curative effect of the three strains of Streptomyces against B. cinerea, the same method was used except that inoculation of B. cinerea at 106 spores ml−1 was realized before the treatment with a spore suspension at 107 spores ml−1 of a strain of Streptomyces sp. In this case, treatment with Streptomyces was realized 0, 2, 4 or 6 h after the inoculation with the strains BC1 of B. cinerea.
All plants were incubated in a growth chamber with a photoperiod of 14 h and maintained at 21 °C with a relative humidity above 80% to favor disease development. The experiment was repeated three times independently, each with three replicates (three plants each with three pruning wounds) per treatment. Lesion expansion on tomato stem was recorded from the 3rd to the 7th day after inoculation (DAI) and AUDPC and protection index were computed as described above.
Statistical analysis
The data were subjected to one- or two-way analysis of variance (ANOVA) using Statistical Package for the Social Sciences (SPSS) version 15 for Windows. When appropriate, the means were compared with the Duncan’s multiple range test (DMRT) and a P < 0.05 was considered as being significant.
Results
In vitro antagonism of Streptomyces spp. against different strains of B. cinerea
The in vitro dual culture assay shows that the three strains of Streptomyces spp. inhibited the mycelial growth of the 41 strains of B. cinerea in the range of 73–100% inhibition on PDA medium after two days of incubation at 21 °C, compared with the control treatment without Streptomyces (Fig. 1). Significant differences in sensitivity to the three strains of Streptomyces sp. were observed between strains of B. cinerea (S. philanthi RM-1-138; F = 4.36, df = 40, 82, P < 0.0001; S. philanthi RL-1-178; F = 3.45, df = 40, 82, P < 0.0001; S. mycarofaciens SS-2-243; F = 3.03, df = 40, 82, P < 0.0001). The strains RM-1-138 and RL-1-178 of S. philanthi completely inhibit the mycelial growth of 31 out of the 41 strains of B. cinerea tested and the strain SS-2-243 of S. mycarofaciens completely inhibited the mycelial growth of 25 strains of B. cinerea. Culture filtrates of each strain of Streptomyces have a strong inhibitory effect on the spore germination of the strains BC1 of B. cinerea (Fig. S1; Supplementary data). This inhibitory effect is very effective (100% inhibition of spore germination) even when the culture filtrate is diluted at 10−3, suggesting a strong antifungal effect of the three strains of Streptomyces spp. tested.
Mycelial growth inhibition of the 41 strains of B. cinerea caused by the strains RM-1-138 (a) and RL-1-178 (b) of S. philanthi and the strain SS-2-243 (c) of S. mycarofaciens revealed by dual culture technique on PDA agar plates and incubated at 21 °C for two days. Values are mean of three replications (±SE). Values with the same letter are not significantly different (ANOVA, P < 0.05; Duncan multiple range test)
To test the effect of volatile compounds produced by Streptomyces spp. on the mycelial growth of B. cinerea, the in vitro inhibitory action of volatiles was tested on ten isolates of B. cinerea having various level of sensitivity to the bacteria based on dual culture assay (Fig. 2). Significant differences between strains of Streptomyces spp. was observed (S. philanthi RM-1-138; F = 63.64, df = 9, 20, P < 0.0001; S. philanthi RL-1-178; F = 92.53, df = 9, 20, P < 0.0001; S. mycarofaciens SS-2-243; F = 51.28, df = 9, 20, P < 0.0001) with an inhibition ranging from 6 to 30% depending both on the strain of B. cinerea and on the strain of Streptomyces spp. Some strains of B. cinerea are significantly less sensitive to Streptomyces than others. The strain E258 of B. cinerea is for instance among the least sensitive strain against the three strains of Streptomyces.
Mycelial growth inhibition of ten strains of B. cinerea caused by volatile substances produced by each of the strains RM-1-138 (a) and RL-1-178 (b) of S. philanthi and the strain SS-2-243 (c) of S. mycarofaciens on PDA agar plates and incubated at 21 °C for two days. Values are mean of three replications (±SE). Values with the same letter are not significantly different (ANOVA, P < 0.05; Duncan multiple range test)
Efficacy of Streptomyces spp. against B. cinerea on tomato
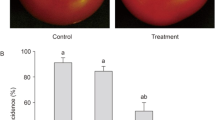
The protective efficacy of the three strains of Streptomyces spp. against the strain BC1 of B. cinerea was evaluated on tomato plants, both on a detached leaf test and on a whole plant test. Two spore concentrations of Streptomyces spp. (106 or 107 spores ml−1) were evaluated. Globally, the highest is the spore concentration of Streptomyces spp. the most effective is the control of B. cinerea on plant organs (Fig. 3). A two-way analysis of variance (strain of Streptomyces spp. × dose of bacteria done on the protection of the biological control agent) was realized independently on detached leaf and on whole plant bioassays. It reveals a Streptomyces strain effect (F = 15.62, df = 2, 18, P < 0.0001) and a dose effect (F = 49.04, df = 1, 18, P < 0.0001) on detached leaf bioassay and a Streptomyces strain effect (F = 9.47, df = 2, 18, P = 0.003) and a dose effect (F = 7.95, df = 1, 18, P = 0.015) on whole plant bioassay, with no interaction effect (F = 0.82, df = 2, 18, P = 0.464 and F = 1.58, df = 2, 18, P = 0.246 for detached leaf and whole plant bioassays, respectively). The strain RM-1-138 of S. philanthi at 107 spores ml−1 exhibits the strongest protection effect against B. cinerea on tomato plants with 57% protection on whole plants and 60% protection on detached leaves. The two other strains of Streptomyces sp. provide a lower protective effect against B. cinerea on tomato.
Protection of tomato plants (in percentage) on whole plant assay (a) and detached leaf assay (b) against the strain BC1 of B. cinerea with the strains RM-1-138 and RL-1-178 of S. philanthi and the strain SS-2-243 of S. mycarofaciens at two spore concentrations (106 and 107 spores ml−1). Values are mean of three repetitions (±SE). To test the hypothesis that the efficacy of a biocontrol agent was not dependent on the strain of Streptomyces spp. and on the dose of bacteria used, a two-way analysis of variance (strain of Streptomyces spp. × dose of bacteria) was realized for each bioassay and the Duncan’s multiple range Test was used to compare the percentage of protection of tomato against B. cinerea. Values with the same letter are not significantly different for each bioassay independently (two-way ANOVA done on protection, P < 0.05; Duncan multiple range test)
Efficacy of the strain RM-1-138 of S. philanthi against three different strains of B. cinerea on tomato
Three isolates of B. cinerea having various levels of in vitro sensitivity to the strain RM-1-138 were tested on tomato plants to estimate the efficacy of protection generated by this strain (Fig. 4). On both bioassays (whole plant test and detached leaf test) and whatever the dose of RM-1-138 applied (106 or 107 spores ml−1), lesions caused by B. cinerea were significantly reduced compared to the control without the biocontrol agent. A two-way analysis of variance (strain of B. cinerea × dose of RM-1-138 on the protection of the biological control agent) was realized for each bioassay. On detached leaf bioassay, analysis reveals a B. cinerea strain effect (F = 5.08, df = 2, 18, P = 0.025) and a dose effect (F = 17.02, df = 1, 18, P = 0.001) with no interaction (F = 0.36, df = 2, 18, P = 0.703). On whole plant bioassay, an absence of B. cinerea strain effect (F = 1.40, df = 2, 18, P = 0.284), a dose effect (F = 4.22, df = 1, 18, P = 0.05) and no interaction effect (F = 0.28, df = 2, 18, P = 0.764) were observed. S. philanti RM-1-138 at 107 spores ml−1 displays a protection of tomato plants comprised between 45 and 52% on whole plant tests and between 44 and 60% on detached leaf tests, depending on the strain of B. cinerea tested.
Protection of tomato plants (in percentage) on whole plant assay (a) and detached leaf assay (b) against three different strains of B. cinerea with the strain RM-1-138 of S. philanthi at two spore concentrations (106 and 107 spores ml−1). Values are mean of three repetitions (±SE). To test the hypothesis that the efficacy of a biocontrol agent was not dependent on the strain of B. cinerea and on the dose of the strain of RM-1-138 of S. philanthi, a two-way analysis of variance was realized and the Duncan’s multiple range test was used to compare the means of the percentage of protection of tomato against B. cinerea. Values with the same letter are not significantly different at P < 0.05
Preventive and curative effect of S. philanthi RM-1-138 against B. cinerea on tomato plant
Treatment of pruning wounds of tomato with a spore suspension of S. philanthi RM-1-138 concomitantly with an inoculation of a spore suspension of B. cinerea gives a protection index of approximately 50% (Fig. 5). When the plants are treated with S. philanthi few hours before the inoculation with B. cinerea the protective effect decreases significantly (ANOVA on the protection index: F = 342.06, df = 3, 8, P < 0.0001). A treatment realized on pruning wounds with S. philanthi, 6 h before the inoculation with B. cinerea tends to favor the development of B. cinerea (protection index = −23%). When the plants are treated with S. philanthi few hours after the inoculation with B. cinerea, the protective effect also decreases significantly (ANOVA on the protection index: F = 172.14, df = 3, 8, P < 0.0001). The protection index attained less than 5% when S. philanthi was inoculated 6 h after the fungal pathogen B. cinerea.
Timing of preventive (a) or curative (b) measures by the strain RM-1-138 of S. philanthi on the protection of tomato plants inoculated with B. cinerea. Values are mean of three replications (±SE). Values with the same letter are not significantly different (ANOVA, P < 0.05; Duncan multiple range test)
Discussion
The three strains of Streptomyces spp. tested in this study had a significant effect on the mycelial growth of all strains of B. cinerea tested on PDA agar plates, suggesting a strong direct antifungal effect. Inhibition of germination of B. cinerea conidia is also achieved by the culture filtrate of the three strains of Streptomyces spp. tested. This result was also observed for other strains of Streptomyces such as the culture filtrate of S. globisporus JK-1 that inhibit the spore germination of Magnaporthe oryzae and reduced its appressorial formation on rice leaves (Li et al. 2011). Moreover, volatile compounds produced by the strains Streptomyces spp. have also an inhibitory effect against B. cinerea mycelial growth. The genus Streptomyces is known to produce volatile antifungal compounds (Vaz Jauri et al. 2016). Volatile compounds produced by S. globisporus JK-1 was for instance able to control Penicillium italicum on Citrus microcarpa and B. cinerea on tomato fruit (Li et al. 2010, 2012). Streptomyces albulus NJZJSA2 also produced volatile compounds effective against Fusarium oxysporum and S. sclerotiorum (Wu et al. 2015).
Testing 41 strains of B. cinerea for their degree of sensitivity to the three strains of Streptomyces spp. revealed that some strains of B. cinerea are less sensitive to the inhibitory effect of antimicrobial compounds produced by these bacteria. Studies highlighting the diversity in sensitivity of plant pathogens to antimicrobial compounds produced by biocontrol agents have been listed by Bardin et al. (2015). However to our knowledge, this study is the first one revealing the diversity of sensitivity of a plant pathogen to volatile compounds produced by a biocontrol agent. Detection of less sensitive isolates to these compounds may indicate a risk of developing a resistance in case of increased selection pressure exerted by the given biocontrol agents. Even if a limited diversity in the sensitivity of the different strains of B. cinerea was observed, further studies are needed to evaluate the capacity of B. cinerea to become resistant to these compounds. Indeed, previous studies have demonstrated that B. cinerea can evolve and become resistant to antibiotic produced by biocontrol agents (Ajouz et al. 2010; Fillinger et al. 2012).
In this study, we demonstrated that spore suspension of the three isolates of S. philanthi and S. mycarofaciens tested in this study can significantly control B. cinerea on leaves and stem of tomato plants. Among the three strains tested, S. philanthi RM-1-138 exhibit the strongest protective efficacy against B. cinerea with 57% protection on whole plant and 60% protection on the detached leaf, suggesting that this strain may have the best potential to be used for protection of tomato grown in greenhouses against gray mold. These results confirm the potential of strains belonging to the genus Streptomyces to control B. cinerea (Ge et al. 2015; Jiang et al. 2016). The best protection of tomato against B. cinerea was observed when spore suspension of S. philanthi were inoculated concomitantly with spores of B. cinerea, given a protection index superior to 50% both on leaves and on the stem. However, when the plants were treated with S. philanthi before or after the inoculation of B. cinerea, the protective effect decreased dramatically. The absence of a preventive effect by S. philanthi suggested that this bacteria is not able to induce a strong systemic resistance in tomato plants and also that the strain may not be able to survive in the plant. This low capacity to survive within the plant could be due to its sensitivity to molecules produced by the plant. It could also be due to the temperature of the tests (21 °C) which is not favorable to the growth of S. philanthi (Boukaew and Prasertsan 2014b). Experiments will be done to test these hypothesis and to measure the ability of this strain to survive in tomato tissues. The absence of curative effect suggests that the bacteria is not able to produce enough amount of antifungal compounds to limit the development of B. cinerea on the plant. It reinforces the hypothesis that this bacteria is not able to survive and to develop on the plant. More work is therefore needed to develop a formulation to consider using this strain preventively or curatively.
Our results demonstrated that the three strains of Streptomyces spp. produce very effective anti-Botrytis substances. The antagonistic mechanism of Streptomyces spp. is therefore probably related to the production of inhibitory antifungal volatile and non-volatile compounds. These results confirm that species of Streptomyces spp. can produce a wide spectrum of antifungal compounds such as antibiotics and volatile compounds (Vaz Jauri et al. 2016). The isolation and the characterization of these substances could lead to the development of effective and stable molecule-based product. Other modes of action could be tested, and a variety of fungal cell wall-degrading enzymes associated to biocontrol activity, such as chitinases and β-1,3-glucanase, are known to be synthesized by Streptomyces (de Boer et al. 1998; Mahadevan and Crawford 1997; Mukherjee and Sen 2006; Vaz Jauri et al. 2016).
In conclusion, the strains of Streptomyces tested in this study may have the potential to control gray mold caused by B. cinerea on tomato but further work is definitely required to enhance its efficacy in planta. For instance improvement in the production process and development of a suitable formulation of the bacteria may result in a more effective product.
References
Ajouz S, Nicot PC, Bardin M (2010) Adaptation to pyrrolnitrin in Botrytis cinerea and cost of resistance. Plant Pathol 59:556–566
Baniasadi F, Bonjar GHS, Baghizadeh A, Karimi Nik A, Jorjandi M, Aghighi S, Farokhi PR (2009) Biological control of Sclerotinia sclerotiorum, causal agent of sunflower head and stem rot disease, by use of soil borne actinomycetes isolates. J Agric Biol Sci 4:146–151
Bardin M, Ajouz S, Comby M, Lopez-Ferber M, Graillot B, Siegwart M, Nicot PC (2015) Is the efficacy of biological control against plant diseases likely to be more durable than that of chemical pesticides? Front Plant Sci 6:1–14
Boukaew S, Prasertsan P (2014a) Suppression of rice sheath blight disease using heat stable culture filtrate of Streptomyces philanthi RM-1-138. Crop Prot 6:1–10
Boukaew S, Prasertsan P (2014b) Factors affecting antifungal activity of Streptomyces philanthi RM-1-138 against Rhizoctonia solani. World J Microbiol Biotechnol 30:323–329
Boukaew S, Chuenchit S, Petcharat V (2011) Evaluation of Streptomyces spp. for biological control of Sclerotium root and stem rot and Ralstonia wilt of chili. BioControl 56:365–374
Boukaew S, Plubrukarn A, Prasertsan P (2013) Effect of volatile substances from Streptomyces philanthi RM-1-138 on growth of Rhizoctonia solani on rice leaf. BioControl 58:471–482
Chung WH, Ishii H, Nishimura K, Fukaya M, Yano K, Kajitani Y (2006) Fungicide sensitivity and phylogenetic relationship of anthracnose fungi isolated from various fruit crops in Japan. Plant Dis 90:506–512
de Boer W, Gunnewiek PJAK, Lafeber P, Janse JD, Spit BE, Woldendorp JW (1998) Antifungal properties of chitinolytic dune soil bacteria. Soil Biol Biochem 30:193–203
Decognet V, Bardin M, Trottin-Caudal Y, Nicot P (2009) Rapid change in the genetic diversity of Botrytis cinerea populations after the introduction of strains in a tomato glasshouse. Phytopathology 99:185–193
Elad Y, Pertot I, Cotes Prado AM, Stewart A (2015) Plant hosts of Botrytis spp. In: Fillinger S, Elad Y (eds) Botrytis—the fungus, the pathogen and its management in agricultural systems. Springer, Berlin, pp 413–486
Errakhi R, Bouteau F, Lebrihi A, Barakate M (2007) Evidences of biological control capacities of Streptomyces spp. against Sclerotium rolfsii responsible for damping off disease in sugar beet (Beta vulgaris L.). World J Microbiol Biotechnol 23:1503–1509
Fillinger S, Ajouz S, Nicot P, Leroux P, Bardin M (2012) Functional and structural comparison of pyrrolnitrin- and iprodione-induced modifications in the class III histidine-kinase bos1 of Botrytis cinerea. PLoS ONE 7(8):e42520
Ge BB, Cheng Y, Liu Y, Liu BH, Zhang KC (2015) Biological control of Botrytis cinerea on tomato plants using Streptomyces ahygroscopicus strain CK-15. Lett Appl Microbiol 61:596–602
Helbig J (2002) Ability of the antagonistic yeast Cryptococcus albidus to control Botrytis cinerea in strawberry. BioControl 47:85–99
Islam MR, Jeong YT, Ryu YJ, Song CH, Lee SY (2009) Identification and optimal culture condition of Streptomyces albidoflavus C247 producing antifungal agents against Rhizoctonia solani AG2-2. Mycobiology 37:114–120
Jarvis WR (1980) Taxonomy. In: Coley-Smith JR, Verhoeff K, Jarvis WR (eds) The Biology of Botrytis. Academic Press, London, pp 1–17
Jiang B, Huang Y, Jia Z, Song S (2016) Streptomyces nobilis C51 suppresses gray mold caused by Botrytis cinerea in tomato. Br Microbiol Res J 16:1–13
Lange L, Sanchez LC (1996) Micro-organisms as a source of biologically active secondary metabolites. In: Copping LG (ed) Crop protection agents from nature: natural products and analogues. R Soc Chem, Cambridge, pp 10–26
Lee PJ, Lee SW, Kim CS, Son JH, Song JH, Lee KY, Kim HJ, Jung SJ, Moon JB (2006) Evaluation of formulations of Bacillus licheniformis for the biological control of tomato gray mold caused by Botrytis cinerea. Biol Control 37:329–337
Li Q, Ning P, Zheng L, Huang J, Li G, Hsiang T (2010) Fumigant activity of volatiles of Streptomyces globisporus JK-1 against Penicillium italicum on Citrus microcarpa. Postharvest Biol Technol 58:157–165
Li Q, Jiang Y, Ning P, Zheng L, Huang J, Li G, Jiang D, Hsiang T (2011) Suppression of Magnaporthe oryzae by culture filtrates of Streptomyces globisporus JK-1. Biol Control 58:139–148
Li Q, Ning P, Zheng L, Huang J, Li G, Hsiang T (2012) Effects of volatile substances of Streptomyces globisporus JK-1 on control of Botrytis cinerea on tomato fruit. Biol Control 61:113–120
Locke T, Fletcher JT (1988) Incidence of benomyl and iprodione resistance in isolates of Botrytis cinerea in tomato crops in England and Wales in 1986. Plant Pathol 37:381–384
Mahadevan B, Crawford DL (1997) Properties of the chitinase of the antifungal biocontrol agent Streptomyces lydicus WYEC108. Enzym Microbial Technol 20:489–493
Mukherjee G, Sen SK (2006) Purification, characterization, and antifungal activity of chitinase from Streptomyces venezuelae P10. Curr Microbiol 53:265–269
Omura S (1992) The expanded horizon for microbial metabolites—a review. Gene 115:141–149
Prabavathy VR, Mathivanan N, Murugesan K (2006) Control of blast and sheath blight diseases of rice using antifungal metabolites produced by Streptomyces sp. PM5. Biol Control 39:313–319
Raza W, Yuan J, Ling N, Huang Q, Shen Q (2015) Production of volatile organic compounds by an antagonistic strain Paenibacillus polymyxa WR-2 in the presence of root exudates and organic fertilizer and their antifungal activity against Fusarium oxysporum f. sp. niveum. Biol Control 80:89–95
Sadeghi A, Hessan AR, Askari H, Aghighi S, Bonjar GHS (2006) Biological control potential of two Streptomyces isolates on Rhizoctonia solani, the causal agent of damping-off of sugar beet. I J Biol Sci 9:904–910
Vaz Jauri P, Altier N, Kinkel LL (2016) Streptomyces for sustainability. In: Castro-Sowinski S (ed) Microbial models: from environment to industrial sustainability, microorganism for sustainability 1. Springer, Berlin, pp 251–276
Wu Q, Bai L, Liu W, Li Y, Lu C, Li Y, Fu K, Yu C, Chen J (2013) Construction of a Streptomyces lydicus A01 transformant with a chit42 gene from Trichoderma harzianum P1 and evaluation of its biocontrol activity against Botrytis cinerea. J Microbiol 51:166–173
Wu Y, Yuan J, Yaoyao E, Raza W, Shen Q, Huan Q (2015) Effects of volatile organic compounds from Streptomyces albulus NJZJSA2 on growth of two fungal pathogens. J Basic Microbiol 55:1104–1117
Yourman LF, Jeffers SN (1999) Resistance to Benzimidazole and dicarboximide fungicides in greenhouse isolates of Botrytis cinerea. Plant Dis 83:569–575
Acknowledgements
The authors would like to thank the Office of the Higher Education Commission for a scholarship to Mr. Sawai Boukaew under the CHE-PhD Scholarship Program (23/2554) and Thailand Research Fund (RTA5780002).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Handling Editor: Jane Debode.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Boukaew, S., Prasertsan, P., Troulet, C. et al. Biological control of tomato gray mold caused by Botrytis cinerea by using Streptomyces spp.. BioControl 62, 793–803 (2017). https://doi.org/10.1007/s10526-017-9825-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-017-9825-9