Abstract
BAC03 is a novel Bacillus velezensis strain previously studied for biological control of scabby diseases caused by Streptomyces scabies. To optimize its efficacy in disease control, different application strategies of BAC03 were investigated in this study, including timing, frequency, and concentrations of BAC03. BAC03 was either used for seed tuber treatment, foliar application or drenching in potting mix infested with S. scabies. Neither foliar application nor seed treatment affected disease severity. BAC03 applied five days before planting significantly reduced S. scabies population and completely suppressed radish scab, but the later BAC03 was applied the less effective it was. BAC03 at 105 CFU cm−3 potting mix or higher concentrations was effective in reducing radish scab. Increasing the frequency of BAC03 application did not increase the efficacy on disease reduction. BAC03 also increased the biomass of radish roots and leaves whether the pathogen was present or not.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptomyces spp. are a group of Gram-positive filamentous bacteria (Loria et al. 1997). Some of these species are pathogenic and cause scabby symptoms on plants, including potato, radish, beet, carrot, turnip, and other tap-root crops (Goyer and Beaulieu 1997; Bignell et al. 2010). Among these pathogens, Streptomyces scabies was the predominant pathogen in most of the potato production areas all over the world (Loria et al. 2008; Bignell et al. 2010). They can be saprophytic when no hosts are available and survive extreme environmental conditions due to their desiccation-resistant spores. This makes Streptomyces spp. become competitive soil inhabitants (Loria et al. 2006), and results in the management of the disease being difficult (Dees and Wanner 2012). In the case of potato common scab, the infection process usually starts from pathogen penetration through natural openings or wounds of potato tubers and roots (Stevenson et al. 2001). Secretion of thaxtomin A (a phytotoxin) by pathogenic Streptomyces spp. facilitates their continuous penetration and nutrient acquisition via inhibition of cellulose synthesis (Fry and Loria 2002). Scab lesions become enlarged as the tuber or root expand (Stevenson et al. 2001).
Management of potato common scab has been considered a tough job (Hiltunen et al. 2009; Dees and Wanner 2012). Many tools have been used for managing scab, such as using resistant cultivars (Douches et al. 2009), maintaining high soil moisture (Lapwood et al. 1973), reducing soil pH (Lacey and Wilson 2001), applying chemical treatments (Davis et al. 1976), crop rotation (Larkin et al. 2011), and organic soil amendments (Lazarovits et al. 1999; Larkin and Tavantzis 2013). However, none of above approaches has given a satisfactory efficacy for scab control. In addition, side effects of some methods (Wharton et al. 2012), limitation of resistance germplasm (Wanner and Haynes 2009), and inconsistent effect of the management depending on locations or seasons (Larkin 2008) are all concerns in the production.
Biological control using beneficial microorganisms offers an addition to traditional management (Lugtenberg and Kamilova 2009). In the management of potato common scab, different microorganisms have been studied, such as Pseudomonas spp. (Singhai et al. 2011; St-Onge et al. 2011), non-pathogenic Streptomyces spp. (Beausejour et al. 2003; Hiltunen et al. 2009), and Bacillus spp. (Han et al. 2005). The genus Bacillus consists of many plant beneficial bacteria (Kloepper et al. 2004), which can favor plant growth by using several tools, such as secreting antimicrobial substances (Ongena and Jacques 2008), promoting plant growth by providing more growth-required nutrition (Spaepen et al. 2009), repelling other unfavorable microorganisms by competing for space and/or nutrients (Fan et al. 2011), or inducing plant disease defense systems (Kloepper et al. 2004). Another important characteristic of Bacillus species is that, compared to other beneficial microorganisms, the endospores of the Bacillus group are more resistant to adverse environmental conditions and can help the bacteria survive in some conditions during storage and transportation (Abriouel et al. 2011).
Bacillus velezensis strain BAC03 (Patent No. WO 2013165607 A1, formerly referred to as B. amyloliquefaciens) was isolated from a soil naturally suppressive to potato common scab (Meng et al. 2012b). It has antimicrobial activity against Streptomyces spp. in vitro (Meng et al. 2012a) and a consistent efficacy in potato common scab suppression in the field in two seasons and two locations (Meng et al. 2013). In addition, a plant growth promotion activity was observed by using this strain (Meng et al. 2016). However, a number of factors can affect the efficacy of biological control. The interactions taking place in the rhizosphere among biocontrol agents, pathogens, plants, as well as other biological and environmental factors are complex and can affect the biocontrol efficacy (Bloemberg and Lugtenberg 2001; Kim et al. 2011). In order to test the efficacy of BAC03, and provide information for large-scale application, the effect of different strategies for BAC03 application on disease suppression of common scab should be determined. The objectives of this study were to analyze the effects of various application strategies on the efficacy of BAC03 in controlling disease caused by S. scabies and study the rhizosphere population dynamics of both BAC03 and S. scabies. This study may offer useful information to aid in application strategy determination for reducing scab.
Materials and methods
Bacterial cultures
Bacillus velezensis FZB42 (previously referred as B. amyloliquefaciens), the active ingredient in commercial biocontrol product RhizoVital42 (ABiTEP GmbH Inc., Berlin, Germany), was obtained from the Bacillus Genetic Stock Center (Columbus, OH, USA). B. velezensis QST713 (formerly referred to as B. subtilis) was obtained from the commercial product Serenade (Bayer CropScience Inc., Monheim, Germany) by culturing the product (powder) in tryptic soy broth (TSB; EMB Chemical Inc., Gibbstown, NJ, USA). All Bacillus strains were cultured on tryptic soy agar (TSA; EMB Chemical Inc.). S. scabies 49173 was obtained from the American Type Culture Collection (Manassas, VA, USA), and cultured on yeast malt extract agar (YME; EMB Chemical Inc.).
Effect of Bacillus velezensis BAC03 on common scab reduction with various application methods
Inoculum preparation and planting
For Bacillus spp. inoculum, the bacterial cultures were grown in TSB at 28 °C on a shaking incubator (Thermo Fisher Scientific Inc., Rockford, IL, USA) at 180 rpm for 48 h in the dark. The concentration of bacterial cells in the liquid culture was determined by dilution plating on TSA. For preparation of S. scabies inoculum, strain 49,173 was streaked on YME and incubated in the dark at 28 °C for two weeks. A spore suspension was prepared by adding 10 ml of sterile distilled water to each culture plate and scraping the colonies using a sterile inoculation loop. The concentration of the spore suspension was determined by dilution plating on YME (Hao et al. 2009).
Potato tuber pieces (cv. ‘Snowden’) with at least one eye were surface disinfested with 1% NaClO for 5 min and rinsed three times with sterile distilled water. The tubers were planted in 3.78-l plastic pots containing soil-less potting mix (ASB Greenworld Inc., New Brunswick, VA, USA) in a greenhouse (Michigan State University greenhouse facility, East Lansing, MI, USA). Growth conditions in the greenhouse were around 18 to 22 °C with a 14-h photoperiod supplemented by light at 200 µmol m−2 s−1.
Radish (‘Cherry Belle’, Burpee Inc. Warminster, PA, USA) seeds were pre-germinated on sterile moist filter paper (No.1, Whatman, Pittsburgh, PA, USA) in a Petri dish by incubating overnight at 25 °C. After germination, seedlings were transplanted into potting mix, with two seedlings per pot, 0.5 cm below the potting mix surface in a 1-l pot, and incubated in a growth chamber (24 °C and 14 h light).
BAC03 application by using seed treatment and foliar spray
The study was conducted on both potato and radish. Potato tuber pieces were soaked in a BAC03 liquid culture (106 CFU ml−1) in TSB for 20 min and then air-dried for 24 h at room temperature before planting. To test foliar spray application, 20 ml of BAC03 liquid culture (106 CFU ml−1) was sprayed with a pump sprayer onto potato leaves starting when the plant shoot height reached 15 cm above the surface of the potting mix. The BAC03 was applied weekly for four weeks. Potting mix was covered with a plastic film to avoid contamination from the foliar spray. For pathogen inoculation, 300 ml spore suspension of S. scabies (107 CFU ml−1) was poured from a beaker on the surface of the potting mix (equivalent to inoculum of 106 CFU cm−3 potting mix) two weeks after plant germination, and this was repeated two weeks later. A negative control for Bacillus application used sterile TSB to replace the BAC03 culture. Five pots with one potato plant each were used for each treatment. Potato tubers were harvested four month after planting and lesion severity on the tuber was rated for lesions and given a severity rating using the 0–5 scale of Hao et al. (2009) where 0 = no symptoms, 1 = 1–10% surface area with superficial or raised lesions, 2 = 11–25% surface area with superficial or raised lesions, 3 = 26–50% surface area with superficial or raised lesions, 4 = more than 50% surface area with superficial or raised lesions or 6–25% pitted lesion area, and 5 ≥ 50% surface area with superficial or raised lesions or >25% pitted area. This trial was done twice.
For radish seed treatment, radish seeds were soaked in a BAC03 liquid culture (106 CFU ml−1) for 20 min and then air-dried for 24 h at room temperature before pre-germination. For the foliar spray test, 10 ml of BAC03 culture broth (106 CFU ml−1) was sprayed onto radish leaves as above ten days after planting, and repeated every three days for a total of four times. The potting mix in the pots was covered with a plastic film to avoid contamination from the foliar spray. A spore suspension of S. scabies (300 ml of 107 CFU ml−1) was applied as a drench on the potting mix (equivalent to inoculum of 106 CFU cm−3 potting mix) five days before planting. Inoculation with TSB only was used as a negative control. Five pots with two radish plants each were used for each treatment. Radishes were harvested six weeks after planting and lesion severity was rated according to the method of Wanner (2004), where where 0 = no lesion, 1 = discrete superficial lesions less than 10 mm in diameter, 2 = coalescing superficial lesions more than 10 mm in diameter, 3 = raised lesions less than 10 mm in diameter, 4 = coalescing raised lesions more than 10 mm in diameter, and 5 = pitted or sunken lesions. The trial was carried out twice.
BAC03 application by potting mix drench at different stages of radish growth
In order to determine the optimal timing of BAC03 application for scab suppression, treatments at different radish growth stages were tested, including two and five days before planting (DBP), as well as 10, 20, and 30 days after planting (DAP). A liquid culture of BAC03 (300 ml of 106 CFU ml−1) was added to the potting mix as a drench to give a final concentration of 105 CFU cm−3 potting mix. Streptomyces scabies was directly applied as a drench on the potting mix (300 ml of 107 CFU ml−1) at 2 DBP. Treatments with S. scabies combined with TSB or TSB-only were used for controls. There were four pots with two radish plants each for each treatment. Disease severity was determined as above (Wanner 2004). The weight of leaves and roots were evaluated separately at harvest (six weeks after planting). The trial was carried out twice.
BAC03 application with different concentrations as potting mix drench on radish
A series of BAC03 concentrations, which included 1 × 104, 5 × 104, 1 × 105, 5 × 105, 1 × 106, and 5 × 106 CFU cm−3 potting mix, were tested for radish scab suppression. Streptomyces scabies (300 ml of 107 CFU ml−1) was inoculated at 2 DBP. The liquid culture of BAC03 was diluted and applied as a drench into the potting mix 10 DAP to attain the designated concentrations. Treatments with S. scabies combined with TSB or TSB-only were used for controls. Four pots with two radish plants each were included for each treatment. The disease rating and biomass assessment were conducted as described above. This experiment was conducted twice.
BAC03 application with different frequencies as potting mix drench on radish
Streptomyces scabies (106 CFU cm−3 potting mix) was inoculated by drenching in potting mix at 2 DBP. BAC03 was applied either once (at 10 DAP), or the same total volume of inoculum was divided and applied for a total of two (at 8 and 12 DAP), three (at 7, 10, and 13 DAP), or four (at 7, 9, 11, and 13 DAP) times. Treatments with S. scabies combined with TSB or TSB-only were used for controls. Four pots with two radish plants each were applied for each treatment. The methods for planting, disease rating, and biomass measurement were as described above. This experiment was conducted twice.
Comparison of BAC03 with B. velezensis QST713 and FZB42
BAC03, QST713 and FZB42 were evaluated for scab reduction in radish. Bacillus spp. were applied as liquid cultures at 10 DAP to a final concentration of 105 CFU cm−3 potting mix. Four pots with two radish plants each were used for each treatment. The methods for planting, disease rating, and biomass measurement were as described above. This experiment was conducted twice.
Population dynamics of S. scabies 49173 and B. velezensis BAC03 in the radish rhizosphere
Inoculation
Radish planting and preparation of S. scabies inoculum were conducted as above. Spore suspension of S. scabies was mixed into potting mix by directly applying the spore suspension as a drench into the mix to get a final concentration of 106 CFU cm−3 potting mix (treatment SS). Five days after S. scabies application, radish was planted. Bacillus velezensis BAC03 liquid cultures were conducted as described above. BAC03 was inoculated at either 5 DBP (treatment B1+SS), or 5 DAP (treatment B2+SS). Controls were BAC03 only (treatment B), S. scabies and TSB (treatment SS), and tryptic soy broth only (treatment TSB). Nine replicates were used for each treatment.
Soil DNA extraction
The radish rhizosphere soil was sampled at 10, 20, and 30 DAP. For sampling, radish plants were carefully dug out from the potting mix in order not to disrupt the root integrity. Two plants from each pot were vigorously shaken by hand for 1 min. Then the roots (hair roots or lower non-edible roots) were placed in a 1.5 ml microcentrifuge tube containing one-milliliter of sterile phosphate buffered saline solution (1×, NaCl 8 g l−1, KCL 0.2 g l−1, Na2HPO4 1.44 g l−1, KH2PO4 0.27 g l−1, pH 7.4), and vortexed (3000 × rpm) for 2 min. The roots were removed and the tube was centrifuged at 20,000×g for 10 min. The supernatant was poured out. One tenth of a gram of precipitate was used for DNA extraction. Total DNA was extracted using a FastDNA Spin Kit for Soil (MP Biomedicals, Solon, OH, USA) following the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction (qPCR) assay
To compare the level of rhizosphere population of both S. scabies and B. velezensis BAC03, the amount of organisms were estimated by measuring their DNA in the rhizosphere soil with qPCR. For detection of S. scabies population, the txtA gene was amplified with the primer set txtA-F (TGCTCAACTCCGTGATCCAGTA) and txtA-R (GGGACACCTCGCGCAGTA) (St-Onge et al. 2011). To quantify the population of B. velezensis BAC03, the lci gene was amplified with a set of primers designed for the lci-F (TGCCTTACTGATGTCTGCCG) and lci-R (CGGCATCTGCTTTCGTTGG) (this study). Reactions were prepared in triplicate in 96-well optical plates using a mix consisting of 10 μl of ABI SYBR Green PCR master mix (2X) (Applied Biosystems, Carlsbad, CA, USA), 2 μl of 1/10 diluted DNA, 0.5 μl of each primer (10 μM), and 7 μl of sterile double distilled water. Reactions containing sterile water served as negative controls for each PCR. External standards for quantification consisted of five concentrations of serially diluted S. scabies and B. velezensis genomic DNA (1, 0.1, 0.01, 0.001, 0.0001 ng μl−1), and were included in triplicate in each PCR run to calculate quantities of S. scabies and B. velezensis in the potting mix samples. The PCR was carried out in a StepOnePlus Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA) using the conditions described by the manufacturer with 45 amplification cycles. To confirm single products, the melting curve and gel electrophoresis were used (St-Onge et al. 2011). This experiment was conducted twice.
Statistical analysis
Data were analyzed using SAS software (version 9.2, SAS Inc., Cary, NC, USA). For plant weight, PROC GLM was used for the analysis of variance, and Fisher’s least significant difference (LSD) multiple comparisons were performed for mean separation of yield where ANOVA showed significant differences. Non-parametric data analysis was used for disease analysis, and Kruskal–Wallis test was performed to compare mean values. If there was no interaction between repeated trials (P > 0.05), data were combined from all trials.
Results
Effect of B. velezensis BAC03 on common scab reduction with various application methods
Foliar spray and seed treatment
Foliar spray with BAC03 did not affect the severity of scab either in potato or radish compared to that of broth-treated plants. In the potato trial, disease scores were around 0.9 for the BAC03-treated, and 0.8 for the non-treated plants (χ 2 = 1.12, df = 1, P > 0.05). In the radish trial, disease scores were 0.7 for BAC03-sprayed, and 0.8 for non-treated plants (χ 2 = 0.89, df = 1, P > 0.05). The disease was not significantly reduced when potato (χ 2 = 2.05, df = 1, P > 0.05) and radish (χ 2 = 1.49, df = 1, P > 0.05) seeds were treated with BAC03 and grown in soil infested with S. scabies.
BAC03 application at different stages
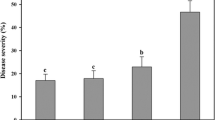
Treatments with a soil drench of BAC03 applied at different stages of radish growth all reduced scab compared to the non-treated control (Fig. 1a, F 6, 49 = 7.17, P < 0.05). The scab disease was not detectable in the treatment with BAC03 applied 5 DBP (Fig. 1a). Biomass assessment showed that treatment with BAC03 applied 10 DAP gave the highest weight of radish leaves, when the root system was well developed and could easily receive the inoculation (Fig. 1b, leaves: F 6, 49 = 9.12, P < 0.05; roots: F 6, 49 = 3.89, P < 0.05).
Effect of application timing of B. velezensis BAC03 on scab (a) and fresh weight of radish (b). BAC03 was applied five and two days (d) before, as well as 10, 20, and 30 days (d) after planting. Treatments with tryptic soy broth (TSB) only and [S. scabies + TSB] (SS) were used as controls. Mean values are the average of two trials, with four replicates (eight radish plants per replicate) each. Error bars represent SE of 16 plants. Mean values were separated by lower-case (for radish leaves) or capital (for radish roots) letters at significance level α = 0.05. Tubers were examined for lesions and given a severity rating using the 0–5 scale (Wanner 2004)
BAC03 application at different concentrations
Treatments with BAC03 at concentrations from 1 × 105 to 5 × 106 CFU cm−3 potting mix all significantly reduced scab in radish compared to the inoculated control (Fig. 2a, F 7, 59 = 8.80, P < 0.05). Radish plants treated with 1 × 105, 5 × 105, 1 × 106, and 5 × 106 CFU cm−3 potting mix had a higher weight of leaves than other treatments and controls (Fig. 2b, F 7, 59 = 39.25, P < 0.05). Radish plants treated with 5 × 104, 1 × 105, 5 × 105, 1 × 106, and 5 × 106 CFU cm−3 potting mix had a higher (edible or swollen) root weight than other treatments and controls (Fig. 2b, F 7, 59 = 129.84, P < 0.05).
Effect of B. velezensis BAC03 applied with different concentrations as a soil drench on scab (a) and fresh weight of radish (b). BAC03 was applied to get final concentrations of 104, 5 × 104, 105, 5 × 105, 106, 5 × 106 CFU cm−3 potting mix. Treatments with tryptic soy broth (TSB) only and [S. scabies + TSB] (SS) were used as controls. Mean values are the average of two trials, with four replicates (eight radish plants per replicate) each. Error bars represent SE of 16 plants. Mean values were separated by lower-case (for radish leaves) or capital (for radish roots) letters at significance level α = 0.05. Tubers were examined for lesions and given a severity rating using the 0–5 scale (Wanner 2004)
BAC03 application with different frequencies
Treatments with a soil drench by BAC03 applied at different frequencies all reduced scab and increased biomass compared to controls (Fig. 3a, b). There were no significant differences in disease severity among treatments with BAC03 at different application frequencies (Fig. 3a, F 5, 37 = 3.09, P < 0.05). However, BAC03 applied more than one time had a higher weight of radish leaves than that with one-time application and the controls (Fig. 3b, F 5, 37 = 63.51, P < 0.05). In the weight assessment of radish swollen roots, all treatments with BAC03 application increased the weight of radish in a variable manner compared to the controls treated with S. scabies and TSB (Fig. 3b, F 5, 37 = 431.78, P < 0.05).
Effect of application frequency of B. velezensis BAC03 as a soil drench on scab (a) and fresh weight of radish (b). Treatments with tryptic soy broth (TSB) only and [S. scabies + TSB] (SS) were used as controls. Mean values are the average of two trials, with four replicates (eight radish plants per replicate) each. Error bars represent SE of 16 plants. Mean values were separated by lower-case (for radish leaves) or capital (for radish roots) letters at significance level α = 0.05. Tubers were examined for lesions and given a severity rating using the 0–5 scale (Wanner 2004)
Comparison of BAC03 with B. velezensis QST713 and FZB42
There was no significant difference in disease suppression among the bacterial strains (Fig. 4a). However, treatment with BAC03 gave a higher weight of radish leaves and (edible or swollen) roots than treatments with FZB42 and QST713 (Fig. 4b, leaves: F 4, 35 = 27.75, P < 0.05; roots: F 4, 35 = 129.67, P < 0.05).
Efficacy comparison of B. velezensis BAC03, B. subtilis QST713, and B. velezensis FZB42 as a soil drench on scab (a) and fresh weight of radish (b). Treatments with tryptic soy broth (TSB) only and [S. scabies + TSB] (SS) were used as controls. Mean values are the average of two trials, with four replicates (two radish plants per replicate) each. Error bars represent SE of 16 plants. Mean values were separated by lower-case (for radish leaves) or capital (for radish roots) letters at significance level α = 0.05. Tubers were examined for lesions and given a severity rating using the 0–5 scale (Wanner 2004)
Population dynamics of S. scabies and B. velezensis BAC03 in the radish rhizosphere
The amounts of genomic DNA of both S. scabies and B. velezensis in the rhizosphere soil of radish treated with BAC03 were determined by a qPCR assay. In the detection of S. scabies, the bacterial population displayed various dynamic responses to different treatments (Fig. 5a). Without BAC03 addition (treatment SS), the levels of S. scabies were around 1.4 μg g−1 potting mix at 10 and 20 DAP, and about 1 μg g−1 potting mix at 30 DAP (Fig. 5a). With the addition of BAC03 before radish planting (treatment B1+SS), the level of S. scabies population was about 0.34 μg g−1 potting mix at 10 DAP, and 0.19 μg g−1 potting mix detected at 30 DAP (Fig. 5a). When BAC03 was applied after radish planting (treatment B2+SS), S. scabies population were determined at 0.94 μg g−1 potting mix at 10 DAP and 0.45 μg g−1 potting mix at 30 DAP. No S. scabies was detected in the controls (treatment B and TSB) (Fig. 5a).
Population dynamics (converted from amount of total DNA in the sample) of S. scabies (a) and B. velezensis BAC03 (b) in radish rhizosphere potting mix analyzed by quantitative polymerase chain reaction. BAC03 was applied either 5 DBP (DBP, treatment B1+SS), or 5 DAP of radish (B2+SS). Controls were BAC03 only (B) applied at 5 DBP, S. scabies only (SS) applied at 5 DBP, and tryptic soy broth (TSB) applied at both 5 DBP and 5 DAP. Values of treatments TSB and B overlap due to the same measurements (all zeros). Error bars represent SE of 18 replicates. Parameters of qPCR assay for txtA: y = −3.419x + 36.737, R 2 = 0.984, E = 96%. Parameters of qPCR assay for lci: y = −3.501x + 35.566, R 2 = 0.981, E = 93%
In the estimation of B. velezensis population in the rhizosphere by detecting lci gene, no DNA was amplified from the treatments SS and TSB (Fig. 5b). When BAC03 was applied after radish planting, the levels were determined 1.44 μg g−1 at ten days to 0.74 μg g−1 at 30 DAP (Fig. 5b, B2+SS). In other two treatments with BAC03 applied before radish planting (B1+SS) and with BAC03 application only (B), the levels of B. velezensis population were lower than that detected in the treatment of B2+SS (Fig. 5b).
Discussion
Results of this study indicated that B. velezensis BAC03 significantly reduces the disease caused by S. scabies. The efficacy of BAC03 for scab control was affected by the application method. Foliar application and seed treatment with BAC03 did not significantly reduce scab severity, while application of BAC03 as a soil drench before planting resulted in reducing radish scab, and this inhibition was reduced as the application delayed. Application of BAC03 at concentrations higher than 105 CFU cm−3 as a drench to the potting mix gave significant reduction of radish scab. Application of BAC03 at various frequencies did not affect scab suppression if the total amount were the same. BAC03 performed equally well compared with two commercial biocontrol products based on Bacillus isolates (B. velezensis QST713 and FZB42).
Understanding how the biocontrol agent interact with plants and pathogens could help to design effective application strategies (Perez-Garcia et al. 2011). Since different biocontrol agents can use different mechanisms (Ongena and Jacques 2008; Kim et al. 2011), the strategy for their application can be species- or strain-specific (Blom et al. 2011). For example, some Bacillus spp. are effective at reducing diseases when applied for plant seed treatments (Zhang et al. 2009; Wharton et al. 2012). This study did not show any effect in scab suppression by using seed treatment or as a foliar spray with B. velezensis strain BAC03. Therefore, it is not recommended to use the above two approaches in scab control through BAC03.
A concentration of 105 CFU cm−3 potting mix was the threshold for significantly reducing scab of the levels tested in this study, which was much lower than that of other Bacillus products that are around 107–108 CFU cm−3 or g−1 soil (Han et al. 2005; Zhang et al. 2009). Such a low concentration requirement makes BAC03 have cost advantage compared to other products if it were used in large-scale application (Spaepen et al. 2009). A critical period for scab development is during radish root expansion, especially at the beginning of development (Bignell et al. 2010). Therefore, scab could be effectively reduced as long as the addition of the biocontrol agent covers this critical disease development stage.
Antagonism and rhizosphere colonization are two major tools used by biocontrol agents (Bloemberg and Lugtenberg 2001; Ongena and Jacques 2008; Fan et al. 2011), including BAC03. We have previously demonstrated that LCI peptide produced by BAC03 is responsible for its antimicrobial activity (Meng et al. 2012a). Moreover, biocontrol agents in the Bacillus group are well documented to produce antimicrobial substance, such as iturins, fengycins, and surfactines (Stein 2005). Colonization is another critical trait for exerting their beneficial effect in the rhizosphere (Fan et al. 2011). Good colonizers are able to compete with plant pathogens for nutrients secreted by the root and for sites that can be occupied on the root (Lugtenberg and Kamilova 2009). When both BAC03 and S. scabies were applied before planting, no disease was observed at harvest. It is possible that BAC03 inhibited and repelled the pathogens, resulting in minimizing the negative influence of S. scabies on radish.
Results of population dynamics in the radish rhizosphere soil detected by qPCR showed various dynamic changes of BAC03 and S. scabies levels in different treatments. BAC03 applied before planting reduced the population of the pathogen S. scabies in rhizosphere soil, indicating that an antagonistic activity play an important role during this period. The effects of BAC03 applied after radish planting was not as good as that with application before planting. We interpreted that BAC03 had a shorter time to interact with the pathogen that had already invaded the host plant. BAC03 levels dropped quickly right after its application. This population survival needs to be considered in disease management. However, BAC03 had an advantage in survival and competition compared with S. scabies. This was demonstrated in the study that even though the concentration of S. scabies applied as a drench was ten times higher than that of BAC03, the detected concentrations of both bacteria were at a similar level. More interestingly, the DNA amount of BAC03 in the treatment with BAC03 only was higher than when BAC03 was inoculated in S. scabies infested soil at 5 DBP, while less than when BAC03 was inoculated in S. scabies infested soil at 5 DAP. The difference between these was that at 5 DAP, root system was initiating, which allowed higher population of BAC03 to colonize.
The optimal application timing by BAC03 in radish growth promotion is different from that in scab disease suppression (Meng et al. 2016), which could because BAC03 uses different strategies in these two processes. In disease suppression, when BAC03 was applied earlier, the biocontrol agent could have an opportunity to interact with, or compete and suppress the pathogen. This benefits BAC03 to colonize the plant rhizosphere. In contrast, growth enhancement is affected by nutrient offered by BAC03, the stage of radish root expansion could be the most critical period for growth promotion. For the same total volume of BAC03, multiple applications provided the nutrients in more efficient way and resulted in a higher biomass of radish. This relationship between rate and response might relate to the survival of the Bacillus strains, since the viable period of BAC03 could be prolonged by dividing the application into several times.
In conclusion, B. velezensis BAC03 displayed an effect in scab disease suppression, and exerted a potential with specific application strategies. This serves a foundation for future applications at large scales.
References
Abriouel H, Franz CM, Ben ON, Galvez A (2011) Diversity and applications of Bacillus bacteriocins. FEMS Microbiol Rev 35:201–232
Beausejour J, Clermont N, Beaulieu C (2003) Effect of Streptomyces melanosporofaciens strain EF-76 and of chitosan on common scab of potato. Plant Soil 256:463–468
Bignell DR, Huguet-Tapia JC, Joshi MV, Pettis GS, Loria R (2010) What does it take to be a plant pathogen: genomic insights from Streptomyces species. Antonie van Leeuwenhoek J Microb 98:179–194
Bloemberg GV, Lugtenberg BJ (2001) Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol 4:343–350
Blom D, Fabbri C, Eberl L, Weisskopf L (2011) Volatile-mediated killing of Arabidopsis thaliana by bacteria is mainly due to hydrogen cyanide. Appl Environ Microbiol 77:1000–1008
Davis JR, Mcmaster GM, Callihan RH, Nissley FH, Pavek JJ (1976) Influence of soil moisture and fungicide treatments on common scab and mineral content of potatoes. Phytopathology 66:228–233
Dees MW, Wanner LA (2012) In search of better management of potato common scab. Potato Res 55:249–268
Douches DS, Coombs J, Hammerschmidt R, Kirk WW, Long C (2009) Kalkaska: a round white chip processing potato variety with common scab resistance. Am J Potato Res 86:347–355
Fan B, Chen XH, Budiharjo A, Bleiss W, Vater J, Borriss R (2011) Efficient colonization of plant roots by the plant growth promoting bacterium Bacillus amyloliquefaciens FZB42, engineered to express green fluorescent protein. J Biotechnol 151:303–311
Fry BA, Loria R (2002) Thaxtomin A: evidence for a plant cell wall target. Physiol Mol Plant Pathol 60:1–8
Goyer C, Beaulieu C (1997) Host range of streptomycete strains causing common scab. Plant Dis 81:901–904
Han JS, Cheng JH, Yoon TM, Song J, Rajkarnikar A, Kim WG, Yoo ID, Yang YY, Suh JW (2005) Biological control agent of common scab disease by antagonistic strain Bacillus sp. sunhua. J Appl Microbiol 99:213–221
Hao JJ, Meng QX, Yin JF, Kirk WW (2009) Characterization of a new Streptomyces strain, DS3024, that causes potato common scab. Plant Dis 93:1329–1334
Hiltunen LH, Ojanpera T, Kortemaa H, Richter E, Lehtonen MJ, Valkonen JP (2009) Interactions and biocontrol of pathogenic Streptomyces strains co-occurring in potato scab lesions. J Appl Microbiol 106:199–212
Kim YC, Leveau J, Gardener BBM, Pierson EA, Pierson LS, Ryu CM (2011) The multifactorial basis for plant health promotion by plant-associated bacteria. Appl Environ Microb 77:1548–1555
Kloepper JW, Ryu CM, Zhang SA (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266
Lacey MJ, Wilson CR (2001) Relationship of common scab incidence of potatoes grown in Tasmanian ferrosol soils with pH, exchangeable cations and other chemical properties of those soils. J Phytopathol 149:679–683
Lapwood DH, Wellings LW, Hawkins JH (1973) Irrigation as a practical means to control potato common scab (Streptomyces scabies) - final experiment and conclusions. Plant Pathol 22:35–41
Larkin RP (2008) Relative effects of biological amendments and crop rotations on soil microbial communities and soilborne diseases of potato. Soil Biol Biochem 40:1341–1351
Larkin RP, Tavantzis S (2013) Use of biocontrol organisms and compost amendments for improved control of soilborne diseases and increased potato production. Am J Potato Res 90:261–270
Larkin RP, Honeycutt CW, Griffin TS, Olanya OM, Halloran JM, He ZQ (2011) Effects of different potato cropping system approaches and water management on soilborne diseases and soil microbial communities. Phytopathology 101:58–67
Lazarovits G, Conn KL, Potter J (1999) Reduction of potato scab, verticillium wilt, and nematodes by soymeal and meat and bone meal in two Ontario potato fields. Can J Plant Pathol 21:345–353
Loria R, Bukhalid RA, Fry BA, King RR (1997) Plant pathogenicity in the genus Streptomyces. Plant Dis 81:836–846
Loria R, Kers J, Joshi M (2006) Evolution of plant pathogenicity in Streptomyces. Annu Rev Phytopathol 44:469–487
Loria R, Bignell DR, Moll S, Huguet-Tapia JC, Joshi MV, Johnson EG, Seipke RF, Gibson DM (2008) Thaxtomin biosynthesis: the path to plant pathogenicity in the genus Streptomyces. Antonie van Leeuwenhoek Int J G 94:3–10
Lugtenberg B, Kamilova F (2009) Plant growth promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Meng QX, Jiang HH, Hanson LE, Hao JJ (2012a) Characterizing a novel strain of Bacillus amyloliquefaciens BAC03 for potential biological control application. J Appl Microbiol 113:1165–1175
Meng QX, Yin JF, Rosenzweig N, Douches D, Hao JJ (2012b) Culture based assessment of microbial communities in soil suppressive to potato common scab. Plant Dis 96:712–717
Meng QX, Hanson LE, Douches D, Hao JJ (2013) Managing scab diseases of potato and radish caused by Streptomyces spp. using Bacillus amyloliquefaciens BAC03 and other biomaterials. Biol Control 67:373–379
Meng QX, Jiang H, Hao JJ (2016) Effects of Bacillus velezensis strain BAC03 in promoting plant growth. Biol Control 98:18–26
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125
Perez-Garcia A, Romero D, De Vicente A (2011) Plant protection and growth stimulation by microorganisms: biotechnological applications of bacilli in agriculture. Curr Opin Biotech 22:187–193
Singhai PK, Sarma BK, Srivastava JS (2011) Biological management of common scab of potato through Pseudomonas species and vermicompost. Biol Control 57:150–157
Spaepen S, Vanderleyden J, Okon Y (2009) Plant growth promoting actions of rhizobacteria. Plant Innate Immun 51:283–320
Stein T (2005) Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol 56:845–857
Stevenson RW, Loria R, Franc DG, Weingartner DP (2001) Compendium of potato diseases, 2nd edn. The American Phytopathological Society, St. Paul
St-Onge R, Gadkar VJ, Arseneault T, Goyer C, Filion M (2011) The ability of Pseudomonas sp. LBUM 223 to produce phenazine-1-carboxylic acid affects the growth of Streptomyces scabies, the expression of thaxtomin biosynthesis genes and the biological control potential against common scab of potato. FEMS Microbiol Ecol 75:173–183
Wanner LA (2004) Field isolates of Streptomyces differ in pathogenicity and virulence on radish. Plant Dis 88:785–796
Wanner LA, Haynes KG (2009) Aggressiveness of Streptomyces on four potato cultivars and implications for common scab resistance breeding. Am J Potato Res 86:335–346
Wharton PS, Kirk WW, Schafer RL, Tumbalam P (2012) Evaluation of biological seed treatments in combination with management practices for the control of seed-borne late blight in potato. Biol Control 63:326–332
Zhang JX, Xue AG, Tambong JT (2009) Evaluation of seed and soil treatments with novel Bacillus subtilis strains for control of soybean root rot caused by Fusarium oxysporum and F. graminearum. Plant Dis 93:1317–1323
Acknowledgements
This project was partially supported by Maine Potato Board, and Michigan State University Project GREEEN. We recognize the great contribution of Drs. Linda Hanson, Dave Douches, and Ray Hammerschmidt in helping and advising the first author in her thesis writing, which is related to the context of this manuscript. The authors are grateful to Samantha Adamowicz, He Jiang, and Carolina Escobar for their assistance in the greenhouse for providing technical support. We thank Y. Bi, H. Liu, and J. Huck for assistance in the lab.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Jesus Mercado Blanco.
Rights and permissions
About this article
Cite this article
Meng, Q., Hao, J.J. Optimizing the application of Bacillus velezensis BAC03 in controlling the disease caused by Streptomyces scabies . BioControl 62, 535–544 (2017). https://doi.org/10.1007/s10526-017-9799-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-017-9799-7