Abstract
Bacterial endophytes offer control against many diseases of crop plants as potential biocontrol agents. Antagonistic bacterial endophytes acting against Phytophthora meadii have been screened from leaf, petiole and root tissues of Hevea brasiliensis. Six bacterial endophytes could exhibit more than 50 % inhibition of P. meadii, among which EIL-2, from disease-free zones showed a maximum of 62.5 % inhibition. The isolate EIL-2 was characterized as Alcaligenes sp. and the other isolates were identified as Pseudomonas aeruginosa. 16S rDNA sequence analysis showed that there existed genetic variation among the five isolates of P. aeruginosa from different tissues of the plant indicating the tissue type adaptation of the isolates. Dual culture technique with endophyte EIL-2 completely arrested the growth of P. meadii when inoculated prior to pathogen. The bioassay with EIL-2 in H. brasiliensis clones, RRII 105 showed 43 % reduction of lesion size on infected leaves whereas in RRIM 600 it was only 30 %.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hevea brasiliensis, the major commercial source of natural rubber, accounts for 99 % of the world’s total natural rubber production. One of the major constraints to H. brasiliensis cultivation is crop loss due to various fungal diseases of leaf, stem and root. Abnormal leaf fall (ALF) is the most destructive disease of H. brasiliensis in India and caused by Phytophthora sp. Extensive defoliation during ALF disease results in considerable loss of 38–56 % yield in different clones. The disease adversely affects growth and bark renewal of the trees (Jacob et al. 1989). Chemical control of the disease was first propounded by Ashplant (1928), who recommended prophylactic spraying of plants with 0.75 % Bordeaux mixture. As an alternative to Bordeaux mixture, copper oxychloride dispersed in agricultural spray oil proved effective for the control of the disease. Interest in biological control has recently intensified due to the realization that fungicides can have adverse environmental effects. The agriculture sector is currently advancing towards environmentally sustainable development holding the increase in productivity along with the protection of the natural resource base for future generations.
Endophytic bacteria live in plant tissues without causing substantive harm or gaining benefit other than residency. Bacterial endophytes in plants reflect a huge genetic and metabolic biodiversity, which is to a great extent not yet explored, but offers a very high application potential. Many promising endophytic bacteria have been reported as biocontrol candidates against plant pathogens (He et al. 2009). The internal tissues of plants provide uniform and safe environment when compared to rhizosphere and phylloplane, where the introduced bacterial population must compete for nutrients and also endure temperature changes and exposure to ultra violet (UV) rays. These advantages envisage the use of endophytic bacteria for more successful biological control of plant diseases.
Bacterial endophytes to control diseases in crop plants is a recent approach and has been found to be very effective in the management of canker disease in poplar (Yin et al. 2011), Phytophthora diseases in black pepper (Aravind et al. 2009) and cocoa (Arnold et al. 2003). The mode of action of endophytes may involve direct antagonism by antibiosis (Sturz et al. 1998) and competition for nutrients, or induction of plant resistance response (M’Piga et al. 1997). Direct antagonism towards pathogens can be attained by the production of antagonistic substances or growth inhibitors, by antibiotics or other antimicrobial metabolites. Lodewyckx et al. (2002) reviewed a wide variety of endophytic bacteria with antagonistic activity against fungal, bacterial and oomycete pathogens. Endophytic Pseudomonas sp. and actinobacteria seem to produce a wide range of antagonistic compounds, including phenazine and pyrrolnitrin antibiotics (Delaney et al. 2001; Coombs et al. 2004).
Although various bacterial strains of Pseudomonas sp. and Bacillus sp. have been effective in suppressing P. meadii in different crops (Suseela and Kumar 2008), no documentation existed on the application of endophytes for the control of P. meadii in H. brasiliensis. Hence there is ample scope for the isolation and screening of bacterial endophytes from H. brasiliensis. The present research aims to screen novel and effective endophytic antagonistic bacterium and hopes to provide an alternative resource for the biocontrol of Phytophthora leaf fall disease of H. brasiliensis. The antagonists were identified through 16S rDNA sequence analysis and the most efficient endophyte was characterized by dual culture technique and bioassay studies.
Materials and methods
Sampling
Tissue samples were collected from root, petiole and leaf of clones RRII 105 and RRIM 600 of H. brasiliensis from five locations in India: (1) Pudukad estate, Thrissur (2) RRII farm, Kottayam (3) New Ambadi estate, Kulasekharam (4) RRS Padiyoor and (5) Taranagar farm, Agarthala.
Isolation and screening of antagonistic bacterial endophytes against P. meadii
Freshly collected samples were cut into sections (1 g) and were surface sterilized by 2 % sodium hypochlorite (Merk, Mumbai, India) for 2–3 min followed by five rinses in sterilized distilled water. All samples were homogenised with mortar and pestle, serially diluted with sterile 0.85 % NaCl, plated on to Tryptic Soy Agar (Hi Media Laboratory Pvt. Ltd. Mumbai, India) and incubated for 48 h at 28 ± 2 °C. Colony forming units (CFU) were counted and expressed as CFU per gram fresh tissue weight. The individual colonies of differing morphologies were picked and re-streaked on fresh plates to obtain pure cultures (Cactano-Anolles et al. 1993).
The endophytic bacterial isolates were screened for their ability to inhibit the growth of the pathogen P. meadii (available from the culture collection of the pathology division, Rubber Research Institute of India). Isolates were assessed by dual culture technique using potato dextrose agar (PDA) plates. Bacterial isolates were streaked on half plates and 5 mm (diameter) P. meadii discs were placed on the other half of the plate parallel to bacterial streak. PDA plates inoculated with P. meadii discs alone served as the control. After seven days of incubation at 28 ± 2 °C, colony diameters and inhibition zones were measured. The percent growth inhibition was calculated using the formula n = (a − b)/a × 100, where n is the percent growth inhibition, a the colony area of uninhibited P. meadii and b is the colony area of treated P. meadii.
Molecular characterization of antagonistic endophytes
Endophytic bacterial isolates showing more than 50 % inhibition against P. meadii were identified on the basis of sequence analysis of 16S rRNA gene. The conserved eubacterial primers used for the amplification of 16S ribosomal DNA were 1) pA-5′-AGAGTTTGATCCTGG CTCAG-3′, 2) pH-5′-AAGGAGGTGATCCA GCCGCA-3′ and the final concentration of the reagents were 1 mM MgCl2, 200 μM dNTP, 100 pmol primers and 50 ng DNA. The PCR reaction was carried out in eppendorf AG22331 Thermal cycler with the following PCR cycle: one cycle at 94 °C for 2 min, followed by 35 cycles at 94 °C for 1 min, 55 °C for 1 min, 72 °C for 2 min, followed by final 2 min incubation at 72 °C. The PCR products were size fractionated on 1 % agarose gel and the bands were excised from the gel and purified using GenElute™ Gel Extraction Kit (Sigma–Aldrich, Steinheim, Germany). Purified 16S rDNA sequences were cloned in pGEMT Easy vector (Promega, Madison, USA), transformed in JM 109 cells (Promega, Madison, USA) and sequenced at Macrogen, Korea. The sequence similarity was analysed by sequences available in the National Center for Biotechnology Information (NCBI) database using BLAST analysis and isolates were identified on the basis of the best match in the database. Sequences of antagonistic bacterial endophytes and reference sequences from NCBI GenBank were aligned using the multiple sequence alignment program ClustalW2. Using the alignment file generated by ClustalW2, phylogenetic analysis was performed in MEGA4 (Tamura et al. 2007). UPGMA (Sneath and Sokal 1973) was used to infer the phylogeny across the data. Bootstrap analysis (1,000 replicates) was also performed to check the reliability of the phylogram (Felsenstein 1985).
Dual culture studies of antagonistic isolate EIL-2 with P. meadii
The antagonist, EIL-2, which was obtained from a disease-free area (Taranagar farm, Agarthala, India) and showed the highest percentage of inhibition against P. meadii was selected for further studies. EIL-2 was evaluated for its antagonistic activity to inhibit the growth of the pathogen P. meadii by conducting dual culture technique on PDA plates at different time intervals of inoculation. EIL-2 was streaked on PDA plates followed by P. meadii at different time intervals such as one, three and six days. On the other hand P. meadii was inoculated on PDA plates followed by antagonist streaked in different time intervals of one, three and six days. PDA plates inoculated with the P. meadii alone served as the control. After seven days of incubation at 28 ± 2 °C, colony diameters and inhibition zones were measured. The percent growth inhibition was calculated as mentioned above.
Bioassay of antagonistic endophyte
The efficacy of the biocontrol agent EIL-2 against P. meadii was studied in a bioassay on tolerant (RRII 105) and susceptible (RRIM 600) clones of H. brasiliensis grown in the greenhouse using one year old polybag plants. The treatments include: (1) EIL-2 broth (2) broth alone and (3) untreated control. Each treatment was applied to 25 plants, arranged in a completely randomized design. The antagonist, EIL2, was inoculated in TSB medium and incubated at 25 °C for three days with constant shaking at 180 rpm, yielding 108 CFU ml−1. Bacterial broth was diluted with water (1:5) and plants were inoculated by foliar spraying (50 ml plant−1) and soil application (50 ml plant−1). Tryptic soy broth treated and untreated plants were served as the controls. On the 7th day post inoculation, the efficacy of the biocontrol agent was assessed by detached leaf technique (Brown and Soepena 1994). P. meadii spores were prepared in oats media yielding 1 × 106 spores ml−1. The abaxial side of the excised leaves from each treatment was inoculated with 25 μl P. meadii spore suspension (six drops in each leaf) and incubated in Petri dishes. Disease development was measured as the average diameter of lesions formed on 3rd day after P. meadii spore inoculation.
Statistical analysis
The data on population density, percentage inhibition of P. meadii and bioassays were analysed by analysis of variance (ANOVA) and treatment means were compared by Duncan’s Multiple Range Test (DMRT). The package used for analysis was SPSS version 10.0.
Results
Isolation and screening of antagonistic bacterial endophytes against P. meadii
Isolation of bacterial endophytes associated with H. brasiliensis was carried out in samples of each root, petiole and leaf tissues collected from two clones and from five locations on the basis of the severity of ALF disease. The clone RRII 105 shows tolerance and the clone RRIM 600 is highly susceptible to ALF disease. Among the five locations, Pudukad estate, Thrissur, is a highly disease-prone area in India and RRS, Padiyoor, RRII farm, Kottayam and New Ambadi estate, Kulasekharam are the moderately disease-prone areas. Taranagar farm, Agartala is a disease-free area.
Various endophytic bacteria were obtained from surface disinfected samples of H. brasiliensis and their population density in various tissues was estimated. Population densities ranged from 1.38 × 105 to 2.63 × 105 CFU g−1 in fresh root tissue, 1.94 × 104 to 3.33 × 104 CFU g−1 in fresh petioles and 4.60 × 103 to 1.36 × 104 CFU g−1 in fresh leaves.
Population densities of bacterial endophytes among the tissues (root, petiole and leaf) of H. brasiliensis showed significant (F2,270) = 2710.59, P < 0.0001) variation. In different locations, irrespective of clones, root tissue supported a higher number of bacterial endophytes and then petiole and leaf tissues (Fig. 1). A significant variation of bacterial population density in root, petiole and leaf tissues was observed between plants from different locations. Endophytic bacterial population densities were not varying among two clones. A total of 252 morphologically different bacterial endophytes were isolated as representative of the different populations.
Out of the 252 isolates tested for antagonism against P. meadii by dual culturing, 42 showed inhibition of P. meadii mycelia ranging from 15 to 62.5 %. More antagonists (19) were isolated from leaf tissues, fourteen antagonists were from root and only nine were from petiole tissues. Four isolates (AIL-4, AIP-1, A2L-4 and EIL-2) strongly inhibited the growth of P. meadii and showed highest percent of mycelia inhibition (62.5 %). The isolates showing more than 50 % inhibition against P. meadii in dual culture studies are given in Table 1.
Molecular characterization of antagonistic endophytes
Data from molecular and phylogenetic analyses were used to taxonomically characterize the antagonists showing more than 50 % inhibition against P. meadii. PCR primers of the 16S rDNA allowed the amplification of product size of around 1,600 bp and 16S rDNA sequences of the antagonists were compared to the sequences of organisms represented in the Gen Bank database. The isolates B2L-10 (Gen Bank ID: HQ641254), A2L-4 (Gen Bank ID: HQ641259), A1P-1 (Gen Bank ID: HQ641258), A2R-1 (Gen Bank ID: HQ641255) and A1L-4 (Gen Bank ID: HQ641256) showed 99 % identity to Pseudomonas aeruginosa and the isolate E1L-2 (Gen Bank ID: HQ641257) showed 99 % identity to Alcaligenes sp. The four best isolates (E1L-2, A2L-4, B2L-10 and A1L-4) were from leaf and others from petiole (A1P-1) and root (A2R-1) tissues of H. brasiliensis. Among the five P. aeruginosa four, A2L-4, A1P-1,A2R-1 and A1L-4, were from highly disease-prone areas and the remaining B2L-10 was from moderately disease-prone areas, RRII farm, Kottayam. The isolate, E1L-2, identified as Alcaligenes sp. was from a disease-free area and has shown maximum (62.5 %) inhibition against P. meadii (Table1).
The highest score sequences were recovered from the database as reference sequences and aligned with the 16S rDNA sequences of the endophytic bacteria from H. brasiliensis plants. Phylogenetic analysis was conducted in MEGA4 based on unweighted pair group method with arithmetic mean (UPGMA) method. Evolutionary distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. All positions containing gaps and missing data were eliminated from the dataset (Complete deletion option). There were a total of 1451 positions in the final dataset. In the dendrogram (Fig. 2) all the P. aeruginosa isolates were clustered together with very high bootstrap value (99 %), where the Alcaligenes sp. formed the outgroup. To assess the relationship between different strains of antagonistic P. aeruginosa, the taxonomic similarity of isolates was compared using dendrogram constructed based on UPGMA. The antagonistic P. aeruginosa isolates A2L-4, A1L-4 (from leaf tissues), A2R-1 (from root tissues) and A1P-1 (from petiole tissues) were collected from H. brasiliensis in Pudukad estate, Thrissur. The isolate AIP-1 showed distinct sequence variation from other isolates and the variations among A2L-4, A1L-4 and A2R-1 were also predictable from the dendrogram. Dendrogram constructed using 16S rDNA sequences of these antagonists demonstrated genetic diversity among P. aeruginosa from leaf, root and petiole tissues of H. brasiliensis.
Phylogenetic tree expressing the relationships of identified endophytic antagonistic bacterial strains based on the 16S rDNA sequences. Numbers above each node are confidence levels (%) generated from 1,000 bootstraps. The scale bar is in fixed nucleotide substations per sequence position. P. aeruginosa strains A2L-4, A2R-1, AIL-4, B2L-10, AIP-1 and Alcaligenes sp. EIL-2 were used in this study. The M22508, HQ652591, Z76672 and JQ773431 are from Gen Bank database as reference strains
Dual culture studies of antagonistic isolate EIL-2 with P. meadii
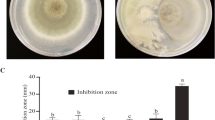
The Alcaligenes isolate EIL-2, the only antagonistic endophyte from a disease-free area, was used for detailed dual culture studies. The antagonistic potential at different inoculation time in both Alcaligenes EIL-2 and P. meadii was observed in dual culturing and the percentage inhibition was up to 62.5 % when the antagonist, Alcaligenes EIL-2, and P. meadii were inoculated simultaneously. The inhibition was increased up to 68 % when P. meadii was inoculated on the 3rd day after EIL-2 inoculation. The complete growth arrest was observed in dual culture plates where P. meadii was inoculated on the 6th day after EIL-2 inoculation (100 % inhibition) (Table 2a).
On the other hand, in the experiment where Alcaligenes EIL-2 was streaked three days after P. meadii inoculation, the percentage of inhibition was decreased up to 13.3 % and then to 8.0 % when Alcaligenes EIL-2 was streaked six days after P. meadii inoculation (Table 2b). The antagonist, Alcaligenes EIL-2, showed effective inhibition when introduced prior to the growth of P. meadii and the inhibition was poor when applied after P. meadii in the dual culture plate.
Bioassay of antagonistic endophyte
The isolate Alcaligenes EIL-2 from disease-free areas was selected for bioassay experiments in H. brasiliensis based on its in vitro inhibition of P. meadii. The polybag clone RRII 105 and RRIM 600 of H. brasiliensis were treated prophylactically with the isolate Alcaligenes EIL2 and significantly (P < 0.05) reduced P. meadii infection was observed on leaves relative to the media and untreated controls (Table 3).
Reductions in diameter of necrotic lesions varied among two clones of H. brasiliensis and more reduction was observed in clone RRII 105 than in RRIM 600. The lesion diameter of P. meadii (lesion size, 3.30 mm) in antagonist-treated leaves of clone RRII 105 was reduced 43 % compared to media treated (lesion size, 5.89 mm) and untreated control (lesion size, 6.30 mm) (F2,147 = 26.98, P < 0.05). In clone RRIM 600, 30 % reduction in lesion size (lesion size, 4.34 mm) was observed in antagonist-treated plants than media-treated (lesion size, 6.28 mm) and untreated (lesion size, 6.57 mm) controls (F2,147 = 11.49, P < 0.05) (Table 3).
Discussion
Hevea brasiliensis is one of the youngest domesticated crops and the source of virtually all the world’s natural rubber production. ALF disease caused by P. meadii is the most destructive disease of rubber in India. The disease occurs annually during southwest monsoon months from June to August. Biological control of plant diseases is an eco-friendly and potential component of integrative pest management (IPM) (Jayaraman et al. 2007; Alexander and Richard 2009). Biocontrol bacteria isolated from rhizosphere and phyllosphere have been extensively studied for the control of pre and post harvest diseases (Valerie et al. 2005; Lingfei and Yanmin 2012). However, their performance may vary due to environmental conditions and poor competition for colonization of ecological niches. These problems can be managed by endophytes since the internal habitat ensures supply of nutrients and protects them from competition with other microorganisms. Despite these advantages, endophytic bacteria have not been as widely explored as rhizobacteria. In the present study, bacterial endophytes were isolated from H. brasiliensis and the antagonistic potential of these endophytes against P. meadii was evaluated.
Endophytic bacteria are ubiquitous among plants and have been isolated from both monocotyledonous and dicotyledonous plants, ranging from woody tree species, such as pear (Whitesides and Spottas 1991), oak (Brooks et al. 1994), citrus plant and scots pine (Pirttilä et al. 2005) to herbaceous crop plants, such as sugar beets (Jacobs et al. 1985), maize (Mclnroy and Kloepper 1995), wheat (Conn and Franco 2004) and rice (Sandhiya et al. 2005). Diversity associated with bacterial endophytes exists, not only in the plant species colonized but also in the colonizing bacterial taxa (Lodewyckx et al. 2002).
In this study the disease tolerance and occurrence in two clones from five locations were considered for sample collection. The presence of endophytic bacteria in the H. brasiliensis was demonstrated by isolating culturable bacteria from its roots, petiole and leaf tissues. The endophytic bacterial population density of plants depends on plant species, plant genotype, plant tissues, growth stage and environmental conditions and the highest bacterial densities are usually observed in the roots and decrease progressively from the stem to the leaves (Quadt-Hallman et al. 1997; Lamb et al. 1996) as observed in the present study.
The high population density in roots indicated the preferential colonization on these tissues by bacterial endophytes. Root is thought to be the preferred site for the bacterial entrance in plants and preferential colonization on root tissues may also be a function of proximity to the soil surface. A detectable difference in the bacterial population density in root, petiole and leaf tissues was observed between plants from different locations (Fig. 1). The differences in the frequency of endophytic bacteria among various tissues suggested that the tissue type has an influence on the population of endophytic bacteria in H. brasiliensis.
The in vitro selection of biological control agents based on antagonistic mechanisms and activity has been reported frequently (Cho et al. 2007). The development of an inhibition halo observed in the growth of P. meadii colonies upon bacterial inoculation might be due to the production of bacterial metabolites. This metabolite might have diffused through the culture medium and suppressed the growth of P. meadii. Dual culture plate assays revealed that 45, 33 and 21 % of the bacterial isolates selected from the leaf, root and petiole respectively inhibited hyphal growth of P. meadii. The variation in the percentage inhibition of pathogen growth might be due to the variation in the amount or the types of inhibitory substances produced, which also might be unstable or poorly diffused into the agar (Whipps 1997; Nielsen et al. 1998; Kim et al. 1999).
A higher number of antagonists was reported from leaf tissues of H. brasiliensis from disease-free areas. The composition of antagonistic bacterial isolates obtained from root, petiole and leaf tissues confirmed the specificity of bacterial communities for certain microenvironments. The roles of antagonistic endophytes in plants of disease free areas and in different plant tissues are not well understood and have to be studied in detail (Table 1).
Analysis based on the sequence of the 16S rRNA gene represents a versatile method for bacterial classification, identification and phylogenetic analysis (Kwon et al. 1997; Sun et al. 2008). The genera Bacillus, Enterobacter, Pseudomonas, Agrobacterium, Alcaligenes, Erwinia, Klebsiella and Serratia have been reported as bacterial endophytes in several crop plants of which Bacillus and Pseudomonas are predominant (Sturz et al. 2000). In this study five of the six selected isolates were identified as P. aeruginosa and a remaining one was identified as Alcaligenes sp. P. aeruginosa have been isolated from different plant tissues, suggesting that these bacteria have developed an evolutionary niche within H. brasiliensis plants. Previous studies have shown that isolates belonging to P. aeruginosa and A. faecalis were reported as endophytes from various plant species. The intracellular colonization of rice seedlings by A. faecalis was reported by You and Zhou (1989). Endophytic P. aeruginosa IISRBP 35 strain from black paper showed inhibition of P. capsisi (Aravind et al. 2009).
The dendrogram showed detectable difference among P. aeruginosa isolates of leaf, petiole and root tissues from the same location (Fig. 2). Also, the dendrogram illustrated that antagonistic isolate from the petiole of H. brasiliensis was more diverse than those isolated from the leaf interior. Similarly, same species that were isolated from different tissues showed difference in their action against P. meadii. P. aeruginosa strains A2L-4 and A1L-4 isolated from the leaf tissues and AIP-1 from petiole tissues of H. brasiliensis showed 62.5 % inhibition against P. meadii while the strain A2R-1 from root tissue showed only 50 % inhibition against P. meadii (Table 1). This is the first molecular investigation of the endophytes of H. brasiliensis plants and it provides unequivocal evidence that members of antagonistic P. aeruginosa from different tissues showed genetic and functional diversity. This confirms earlier observations based on endophytes from potato tuber. According to Sturz et al. (1999), bacterial isolates of the same endophytic species collected from the outermost layer of potato tuber expressed greater antibiosis activity against three Fusarium sp. and Phytophthora infestans than strains isolated from deeper tuber layer. A high degree of microenvironment specificity was evidenced in P. aeruginosa isolates from different tissues. The phenotypic and genotypic diversity which was found in natural populations and observed in the study of Phytophthora nicotianae antagonists offers a tremendous resource for the improvement of biological control strains (Rademaker et al. 1998). Genetic differences and variations in antagonistic activity within the same bacterial species from different tissues indicated the tissue type bacterial adaptations in H. brasiliensis. This study provided the basis for understanding how P. aeruginosa strains associated with different tissues of H. brasiliensis and this information can be used for further examining the potential of bacteria from each tissue for their ability as biocontrol agents.
The antagonistic isolate Alcaligenes EIL-2, the only isolate from disease-free zones, exhibiting 62.5 % P. meadii inhibition was selected for the further evaluations. Observations on the inhibitory response suggested that Alcaligenes EIL-2 produced a diffusible inhibitory substance into the medium that inhibited branching tips of hyphae along the edge of a colony. The antagonistic potential of Alcaligenes EIL-2 was compared at different time intervals of inoculation and it revealed that establishment of Alcaligenes EIL-2 prior to establishment of P. meadii gave more antagonistic potential in dual culture plate (Table 2a and b). The timely application of antagonists before the onset of P. meadii growth offered better biocontrol potential. The results of the present study indicated that versatile endophytic bacterial inhabitants of the H. brasiliensis could act as antagonists against P. meadii. Furthermore the sampling and detection approach adopted in this attempt proved to be an effective method for screening biocontrol agents.
Endophytic bacterial species are known for their beneficial association with the host plants and for their antagonistic activity against plant pathogens. Endophytic bacteria have been shown to control Fusarium oxysporum f. sp. pisi on pea (Benhamou et al.1996), Rhizoctonia solani and Sclerotium rolfsii on bean (Pleban et al. 1997) and Ceratocystis fagacearum on oak (Brooks et al. 1994). Biological control provides an attractive and ecofriendly option to control or suppress the development of Phytophthora diseases. Numerous studies have examined biological control of P. palmivora in cocoa, using microbial antagonists such as Bacillus spp., Aspergillus tamarii, A. gigentus, Botryodiplodia theobromae, Penicillium purpurescens and Pseudomonas fluorescens, with some success (Galindo 1992).
In vitro tests for antagonism served as a good screening procedure to identify effective strain. However, it is important to point out that quite often there is no correlation between in vitro inhibition and field trials (Fravel 1988). In the present study the bioassays of antagonistic agent Alcaligenes EIL-2 on polybag plants of H. brasiliensis in greenhouse showed significant reduction in disease intensity in treated plants compared to the control. The study demonstrated that the leaves of tolerant clone RRII 105 and susceptible clone RRIM 600 inoculated with antagonist showed lesser lesion size compared to untreated control during P. meadii infection (Table 3).
This established that antagonism displayed by the endophytic Alcaligenes sp against P. meadii may be used to control Phytophthora leaf fall disease in fields after intense trials and evaluations. The knowledge generated through this study on genotypic and phenotypic diversity of the endophytes and also on the possibility of endophytic control of the Phytophthora disease provides a strong foundation and enormous resources for future studies in the biological control of Phytophthora leaf fall disease in H. brasiliensis.
References
Alexander RM, Richard AS (2009) Biological control of Radopholus similis in banana by combined application of the mutualistic endophyte Fusarium oxysporum strain 162, the egg pathogen Paecilomyces lilacinus strain 251 and the antagonistic bacteria Bacillus firmus. BioControl 54:263–272
Aravind R, Kumar A, Eapen SJ, Ramana KV (2009) Endophytic bacterial flora in root and stem tissues of black pepper (Piper nigrum L.) genotype: isolation, identification and evaluation against Phytophthora capsici. Lett Appl Microbiol 48:64–67
Arnold AE, Majia LC, Kyllo D, Rojas EI, Maynard Z, Robbins N, Herre A (2003) Fungal endophytes limit pathogen damage in a tropical tree. Proc Natl Acad Sci USA 100:15649–15654
Ashplant HT (1928) Bordeaux and burgundy spraying mixtures. Scientific Department Bulletin, United Planter’s Association of South India, Coonoor, TamilNadu, India
Benhamou N, Kolepper JW, Quadt- Hallman A, Tuzun S (1996) Induction of defence related ultrastuctural modifications in pea root tissues inoculated with endophytic bacteria. Plant Physiol 112:919–929
Brooks DS, Gonzalez CF, Appel DN, Filer TH (1994) Evaluation of endophytic bacteria as potential biological control agents for oak wilt. Biol Control 4:373–381
Brown AE, Soepena H (1994) Pathogenicity of Colletotrichum acutatum and C. gloeosporioides on Hevea spp. Mycol Res 98:264–266
Cactano-Anolles G, Faueluken G, Beber WD (1993) Optimizations of surface sterilization for legume seed. Crop Sci 87:561–568
Cho KM, Hong SY, Lee SM, Kim YH, Kahng GG, Lim YP, Kim H, Yun HD (2007) Endophytic bacterial communities in ginseng and their antifungal activity against pathogens. Microb Ecol 54:341–351
Conn VM, Franco CMM (2004) Analysis of the endophytic actinobacterial population in the roots of wheat (Triticum aestivum L.) by terminal restriction fragment length polymorphism and sequencing of 16S rRNA clones. Appl Environ Microbiol 70:1787–1794
Coombs JT, Michelsen PP, Franco CMM (2004) Evaluation of endophytic actinobacteria as antagonists of Gaeumannomyces graminis var. tritici in wheat. Biol Control 29:359–366
Delaney SM, Mavrodi DV, Bonsall RF, Thomashow LS (2001) phzO, a gene for biosynthesis of 2-hydroxylated phenazine compounds in Pseudomonas aureofaciens 30–84. J Bacteriol 183:318–327
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fravel DR (1988) Role of antibiosis in the biocontrol of plant diseases. Annu Rev Phytopathol 26:75–91
Galindo JJ (1992) Prospects for biological control of cacao. In: Keane PJ, Putter CA (eds) Cocoa pest and disease management in Southeast Asia and Australasia. FAO Plant Production and Protection Paper, Rome, Italy pp 31–36
He RL, Wang GP, Liu XH, Zhang CL, Lin FC (2009) Antagonistic bioactivity of an endophytic bacterium isolated from Epimedium brevicornu Maxim. Afr J Biotechnol 8:191–195
Jacob CK, Edathil TT, Idicula SP, Jayarathnam K (1989) Effect of abnormal leaf fall disease caused by Phytophthora sp. on the yield of rubber tree. Indian J Nat Rubber Res 2:77–80
Jacobs MJ, Bugbee WM, Gabrielson DA (1985) Enumeration, location, and characterization of endophytic bacteria within sugar beet roots. Can J Microbiol 63:1262–1265
Jayaraman J, Parthasarathi T, Radhakrishnan NV (2007) Characterization of a Pseudomonas fluorescens strain from tomato rhizosphere and its use for integrated management of tomato damping-off. BioControl 52:683–702
Kim BS, Moon SS, Hwang BK (1999) Isolation, identification and antifungal activity of a macrolide antibiotic, oligomycin A, produced by Streptomyces libani. Can J Bot 77:850–858
Kwon SW, Go SJ, Kang HW, Ryu JC, Jo JK (1997) Phylogenetic analysis of Erwinia species based on 16S rRNA gene sequences. Int J Syst Bacteriol 47:1061–1067
Lamb TG, Tonkyn DW, Kluepfel DA (1996) Movement of Pseudomonas aureofaciens from the rhizosphere to aerial plant tissue. Can J Microbiol 42:1112–1120
Lingfei X, Yanmin D (2012) Effects of yeast antagonist in combination with UV-C treatment on postharvest diseases of pear fruit. BioControl 57:451–461
Lodewyckx C, Vangronsveld J, Porteous F, Moore ERB, Taghavi S, Mezgeay M, der Lelie D (2002) Endophytic bacteria and their potential applications. Crit Rev Plant Sci 21:583–606
Mclnroy JA, Kloepper JW (1995) Population dynamics of endophytic bacteria in field-grown sweet corn and cotton. Can J Microbiol 41:895–901
M’piga P, Bélanger RR, Paulitz TC, Benhamou N (1997) Increased resistance to Fusarium oxysporum f. sp. radicis-lycopersici in tomato plants treated with the endophytic bacterium Pseudomonas fluorescens strain 63–28. Physiol Mol Plant Path 50:301–320
Nielsen MN, Serensen J, Fels J, Pedersen HC (1998) Secondary metabolite- and endochitinase-dependent antagonism toward plant-pathogenic microfungi of Pseudomonas fluorescens isolates from sugar beet rhizosphere. Appl Environ Microbiol 64:3563–3569
Pirttilä AM, Pospiech H, lakkanen H, Myllyalä R, Pospiech H, Hohtola A (2005) Seasonal variations in location and population structure of endophytes in buds of Scots pine. Tree Physiol 25:289–297
Pleban S, Cherian L, Chet I (1997) Chitinolytic activity of an endophytic strain of Bacillus cereus. Lett Appl Microbiol 25:284–288
Quadt-Hallman A, Benhamou N, Kloepper JW (1997) Bacterial endophytes in cotton: mechanisms of entering the plant. Can J Microbiol 43:557–582
Rademaker JLW, Louws FJ, de Bruijn FJ (1998) Characterization of the diversity of ecologically important microbes by rep-PCR genomic fingerprinting. In: Akkermans ADL, van Elsas JD, de Bruijn FJ (eds) Molecular microbial ecology manual. Kluwer Academic, Dordrecht, The Netherland pp 1–27
Sandhiya GS, Sugitha TCK, Balachandar D, Kumar K (2005) Encophytic colonization and in planta nitrogen and nitrogen fixation by a diazatrophic Serratia sp. in rice. Indian J Exp Biol 43:802–807
Sneath PHA, Sokal RR (1973) Numerical taxonomy. Freeman, San Francisco, USA
Sturz AV, Christie BR, Matheson BG (1998) Association of bacterial endophyte populations from red clover and potato crops with potential for beneficial allelopathy. Can J Microbiol 44:162–167
Sturz AV, Chiristie BR, Matheson BG, Arsenault WJ, Buchanan NA (1999) Endophytic bacterial communities in the periderm of potato tubers and their potential to improve resistance to soil born plant pathogens. Plant Pathol 48:360–369
Sturz AV, Christie BR, Novak J (2000) Bacterial endophytes: potential role in developing sustainable systems of crop production. Crit Rev Plant Sci 19:1–30
Sun L, Qiu F, Zhang X, Dai X, Dong X, Song W (2008) Endophytic bacterial diversity in rice (Oryza sativa L.) roots estimated by 16S rDNA sequence analysis. Microb Ecol 55:415–424
Suseela BR, Kumar A (2008) Effect of rhizobacteria on Phytophthora meadii, Fusariumoxysporum f.sp. vanillae and Colletotrichum vanillaeinfecting vanilla. J Biol Control 22:33–41
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Valerie G, Carole M, Hani A, Russell JT (2005) Antagonist microorganisms with the ability to control Pythium damping-off of tomato seeds in Rockwool. BioControl 50:771–786
Whipps JM (1997) Developments in the biological control of soil-borne plant pathogens. Adv Bot Res 26:1–134
Whitesides SK, Spottas RA (1991) Frequency, distribution and characteristics of endophytic Pseudomonas syringae in pear trees. Phytopathology 81:453–457
Yin XT, Xu LN, Xu L, Fan SS, Liu ZU, Zhang XY (2011) Evaluation of the efficacy of endophytic Bacillus amyloliquefaciens against Botryosphaeria dothidea and other phytopathogenic microorganisms. Afr J Microbiol Res 5:340–345
You CB, Zhou FY (1989) Non-nodular endorhizospheric nitrogen fixation in wetland rice. Can J Microbiol 35:403–408
Acknowledgments
The authors are highly thankful for the facilities provided at Rubber Research Institute of India and Mahatma Gandhi University, Kottayam, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Monica Hofte
Rights and permissions
About this article
Cite this article
Abraham, A., Philip, S., Kuruvilla Jacob, C. et al. Novel bacterial endophytes from Hevea brasiliensis as biocontrol agent against Phytophthora leaf fall disease. BioControl 58, 675–684 (2013). https://doi.org/10.1007/s10526-013-9516-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-013-9516-0