Abstract
Release of a biocontrol agent in New Zealand is typically preceded by non-target testing of native or valued species. Nevertheless, if both the target pest and the natural enemy are very different from any native fauna, then there may be no scientific justification for host testing. Gonatocerus ashmeadi (Girault) (Hymenoptera: Mymaridae) is being considered as a biocontrol agent for glassy winged sharpshooter, Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae), should the pest arrive. An assessment of the potential impact of G. ashmeadi on New Zealand’s Cicadellidae and Membracidae, from published literature data, indicates that none of these insects is at risk, as their eggs will not be recognised by the parasitoid because either their size or location places them outside the parasitoid’s search pattern. Consequently, there is no scientific case for any non-target host-testing to be carried out in containment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
New Zealand regulates the introduction of new classical biological control agents through the Hazardous Substances and New Organisms Act 1996. An applicant first seeks approval from New Zealand’s Environmental Protection Authority (EPA), which replaced the Environmental Risk Management Authority (ERMA) in July 2011, to import a selected agent into containment. Once in containment, the EPA expects that the parasitoid’s potential impact against possible non-target species will be experimentally measured. When experiments have assessed the level of non-target attack, approval may be sought for release. The EPA typically relies on the results of host-testing experiments to assess the environmental risks posed by new biocontrol agents, and to help weigh the perceived economic and environmental benefits of the agent against its potential environmental costs.
In recent decades, host-testing procedures and protocols in containment have been placed on an increasingly sound scientific footing (e.g. van Driesche and Reardon 2004; Kuhlmann et al. 2006; van Lenteren et al. 2006), and the scientific basis of host-testing has become an integral part of the risk analysis process in New Zealand. Nevertheless, it is imperative that host-testing remains tied to its scientific roots, and does not become a bureaucratic requirement for its own sake. This is because, occasionally, both the target host pest and the co-evolved natural enemy species being considered for importation are so different from any native fauna in New Zealand that there is no scientific justification for host testing. This scenario is most likely to occur when the target insect is only extremely distantly related, both phylogenetically and ecologically, to any New Zealand native insects.
The glassy winged sharpshooter (GWSS), Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae) and the bacterium (Xylella fastidiosa Wells et al. 1987) that it vectors, are both potentially serious pests of grapes, citrus and several species of endemic trees in New Zealand. Should GWSS establish in New Zealand, the only feasible and sustainable long-term control measure will be to introduce one or more natural enemies in a classical biological control (CBC) programme. GWSS is indigenous to south-eastern USA and north-eastern Mexico (Turner and Pollard 1959). Following its establishment in California in the late 1980s, the California Department for Food and Agriculture initiated a long-term biological control programme, and Gonatocerus ashmeadi (Girault) (Hymenoptera; Mymaridae), a solitary egg endoparasitoid, was identified as a key natural enemy of GWSS (Irvin and Hoddle 2006). Gonatocerus ashmeadi is also indigenous to south-eastern USA and north-eastern Mexico (Vickerman et al. 2004), where it is a specialist egg parasitoid of GWSS and a few other species in the cicadellid tribe Proconiini. GWSS invaded Tahiti in 1999, Hawaii in 2004, Easter Island in 2005 and Rarotonga (Cook Islands) in 2007 (Petit et al. 2008). Gonatocerus ashmeadi was introduced into Tahiti in 2004, and was self-introduced into Hawaii (probably with GWSS eggs) also in 2004. In both places, the parasitoid rapidly suppressed GWSS populations (Grandgirard et al. 2008). In these intensive studies, which included examination of non-target species, G. ashmeadi has never been recorded to attack any species outside the Proconiini, either in its region of origin, or in those areas where it has been introduced as a biocontrol agent.
As a result of the effectiveness of G. ashmeadi against GWSS in California and French Polynesia, and of biological studies of several other species of GWSS egg parasitoids in California, G. ashmeadi is considered to be the optimum candidate for release into New Zealand if (or when) GWSS arrives (Mark Hoddle, University of California, USA, pers. comm.). In this paper, evidence is presented from overseas biocontrol programmes that the known biological features of GWSS and G. ashmeadi allow the environmental risk posed by G. ashmeadi to be adequately assessed through a literature analysis alone. Together with the known biology of the New Zealand fauna, the results demonstrate that host-testing of non-target species in New Zealand is not justified.
Methods
It is rarely practical or possible to investigate in containment the response of a parasitoid to every non-target species it might encounter in a new country, and so a key part of assessing the risk posed by any potential biocontrol agent is to develop an appropriate list of non-target host species to expose to the enemy. The first criterion is usually to select species that are most closely related to the target host. More distantly related species are then selected if they have biological or ecological characteristics that are similar to that of the host, or if they are of particular socio-economic value (Kuhlmann et al. 2006). This approach has been used recently in New Zealand to select non-target species for exposure to potential classical biocontrol agents (Charles and Dugdale 2011, Berndt et al. 2009).
Here, the science literature was reviewed to examine the potential non-target effects of G. ashmeadi if introduced as a classical biocontrol agent against GWSS. New Zealand insects were examined against two broad criteria: (i) the phylogenetic relatedness of GWSS to New Zealand fauna, and (ii) the biological and ecological requirements of hosts required for the survival of G. ashmeadi.
Phylogenetic relatedness of the New Zealand fauna to GWSS
All known Mymaridae are exclusively parasitoids of the eggs of other insects. The cosmopolitan genus Gonatocerus, with about 280 species worldwide, is one of the largest in the Mymaridae, but the confirmed hosts of the genus are restricted to the Cicadellidae and Membracidae (Noyes 2011; Noyes and Valentine 1989; Matthews 1986). This means that the potential host-range of G. ashmeadi in New Zealand is most likely to be limited to these two families and so all species of Cicadellidae and Membracidae in New Zealand were assessed for their phylogenetic relatedness to GWSS.
Biological and ecological requirements for the survival of G. ashmeadi
The two criteria that have been shown to be critical for predicting the host range of G. ashmeadi are (i) the size of the host and its eggs and (ii) the egg laying biology and ecology of the host (Grandgirard et al. 2007). These two criteria were assessed for the Cicadellidae and Membracidae in New Zealand as follows:
Size criteria
GWSS is a large leafhopper. Females are 12–14 mm long, and lay large eggs (c. 2.5–3 mm long and c. 0.53 mm wide) in obvious clutches usually of eight–nine per clutch, although clutches up to 30 eggs have been found (Boyd and Hoddle 2007). The eggs of all the known hosts of G. ashmeadi are also rather large. Adult female G. ashmeadi are also relatively large. At about 2-mm long, they are two to four times the size of most New Zealand Mymaridae (Noyes and Valentine 1989). In California, G. ashmeadi does not attack either the green sharpshooter, Draeculacephala minerva Ball or the blue-green sharpshooter, Graphocephala atropunctata (Signoret). Both species are about half the size of GWSS, with correspondingly smaller eggs that are probably of insufficient size to support development of G. ashmeadi (Boyd and Hoddle 2007). Hence, any New Zealand leafhopper less than 7 mm long was rejected as a potential host of the parasitoid, because (i) the eggs would not be recognised as hosts by G. ashmeadi adults, and (ii) even if the eggs were attacked, they would likely be too small to support the development of G. ashmeadi larvae to adults.
Egg laying biology and ecology criteria
A posteriori non-target impact studies, in both the laboratory and field, on indigenous cicadellids in California have shown the importance of the egg laying biology of the hosts. In addition to the size constraint discussed above (whereby G. ashmeadi attacked only eggs of similar size to those of GWSS), G. ashmeadi also attacked only eggs that were laid in clusters on the undersides of leaves of trees and bushes (Boyd and Hoddle 2007). Eggs from other species, laid within leaf tissue or singly on stems, bark or leaves, or in grasses were not attacked. This knowledge was used to develop a prediction matrix for assessing the risk posed by G. ashmeadi to native cicadellids in Tahiti (Grandgirard et al. 2007). The risk of the parasitoid attacking any eggs laid on or in grasses, or in the stems of bushes and trees, was predicted to be low. The risk of attack of single eggs laid anywhere was also low. Conversely, the risk of attack of aggregated clutches of eggs on the underside of leaves on trees and bushes was high (Grandgirard et al. 2007).
Although the egg laying biology of many of New Zealand’s Cicadellidae (especially the endemic species) is unknown, it can usually be inferred from either known collection records (which provide details of habitat and possible host plants) or known details of related species from elsewhere in the world. Hence, in New Zealand, the risk of attack by G. ashmeadi of any leafhopper that inhabited grasses, sedges, reeds or other low lying flora (such as bracken or moss) was predicted to be low. The risk would also be low if eggs were laid on stems of trees, or singly in the veins of leaves. Conversely, any species that laid groups of eggs on the undersides of leaves would potentially be at risk of attack by G. ashmeadi.
Results
Phylogenetic relatedness of the New Zealand fauna to GWSS
Homalodisca vitripennis (= coagulata (Say)) lies within the Hemiptera; Membracoidea (= Cicadelloidea auct.); Cicadellidae (the leafhoppers); Cicadellinae; Proconiini (the sharpshooters). The systematics of the Cicadellidae are well studied at a global scale, which means that genera and species within this large family can usually be confidently assigned to lower sub-familial and tribal levels. The New Zealand fauna has also been extensively studied in recent years, providing confidence that there are no significant gaps in our knowledge of the subfamilies present in New Zealand (Larivière et al. 2010). A study of the phylogeny of the major lineages of the Membracoidea based on molecular data (especially 28S rDNA sequences) supported a clade (Cicadellinae) that included only the Cicadellini and Proconiini (Dietrich et al. 2001).
Examination of the New Zealand fauna, from genus level upwards, shows that:
-
1.
There are no species of the genus Homalodisca in New Zealand or Australia.
-
2.
The ‘Old World’ tribe Proconiini (to which Homalodisca belongs) does not occur anywhere in Australasia.
-
3.
The Cicadellinae are a Western Hemisphere group of leafhoppers that are, again, completely absent from New Zealand. The subfamily Cicadellinae includes the economically important ‘sharpshooters’ that transmit the bacterial plant pathogen X. fastidiosa.
-
4.
The very large family Cicadellidae has about 25,000 species worldwide and is represented in New Zealand by ten subfamilies with 78 described species (Larivière et al. 2010). Of these species, 51 are endemic, 11 are native (i.e. they also occur elsewhere, but have been established in New Zealand for a considerable time) and 16 are adventive (i.e. they are exotic, relatively recent arrivals) (Table 1). Some of the species are common and/or widespread, others are rare and/or extremely localised. The family Membracidae (the only other family that is attacked by any species of Gonatocerus) is represented in New Zealand by only one adventive species (Table 1).
Table 1 Summary of the numbers of species and the size of adult females in the subfamilies of Cicadellidae and Membracidae present in New Zealand. See Larivière et al. (2010) for complete species list
Hence, there are no representatives in New Zealand in any of the three most closely related taxa (genus (Homalodisca), tribe (Proconiini) or subfamily (Cicadellinae)) to GWSS. This means that there are no known hosts or potential host groups of G. ashmeadi among the New Zealand leafhoppers such that none is more likely to be attacked by G. ashmeadi than any other, at least on the basis of relatedness.
Biological and ecological requirements for the survival of G. ashmeadi
Literature of all of the New Zealand species of Cicadellidae and Membracidae was examined for the biological and ecological attributes necessary for survival of G. ashmeadi.
Size criteria
From female body sizes provided in taxonomic descriptions of the 78 species of Cicadellidae and one Membracidae in New Zealand (Larivière et al. 2010), 70 species, including 56 endemic/native and 14 adventive species in the Deltocephalinae, Iassinae, Macropsinae, Tartessinae, Typhlocybinae, Xestocephalinae and Centrotinae, have a maximum body length of less than 6.5-mm (Table 1). Some of the endemic species in the Eupelicinae and Ulopinae appear to be much larger than the other New Zealand species, but this is misleading because all of these species have an extraordinarily elongated vertex (head), which can be up to 5 mm long in Paradorydium westwoodi (White) (Eupelicinae). The female abdomen is a more appropriate proxy for egg size, and when body size is corrected for head length, only five species are longer than 7 mm and so might conceivably lay eggs of sufficient size to be accepted by G. ashmeadi females and to support the development of G. ashmeadi larvae (Table 2). The largest species of Cicadellidae in New Zealand is the exotic Euacanthella palustris (Evans), females of which are up to 8.5 mm long (Knight 1974).
Egg laying biology and ecology criteria
Many of New Zealand’s endemic cicadellids are uncommonly collected, and details of their biology are unknown. Nevertheless, the life-histories of these species can usually be inferred from habitat collection data in New Zealand or from field data from related species elsewhere in the world, such that the egg laying biology of the New Zealand species can be predicted.
The Deltocephalinae is the largest subfamily of the Cicadellidae with more than 6,000 species worldwide, most of which are specialist feeders on grasses (Poaceae) or sedges (Cyperaceae) (Zahniser and Dietrich 2010). These are environments in which G. ashmeadi does not search for hosts. All of the New Zealand species conform to this generalisation. The 13 endemic/native species of Athysanini are found predominantly or exclusively in habitats dominated by grass or tussock, and the large, cosmopolitan and taxonomically rather uniform genus Balclutha (with about 100 species, three of which are in New Zealand) are almost universally Gramineae feeders and inhabit grasslands (Knight 1987). Balclutha (? viridinervis) (which is not present in New Zealand) was collected in the Society Islands and considered not to be at risk from G. ashmeadi (Grandgirard et al. 2007). Two Scaphetus species are the only arboreal Deltocephalines (Table 1).
The exotic Euacanthella palustris is the only species of Euacanthellinae in New Zealand. It has a wide host range in many families and is mostly found in lowland coastal regions of New Zealand (Larivière et al. 2010). In Australia, it is found in grasslands and pastures (Fletcher 2009). The New Zealand Eupelicinae are currently represented by up to eight species (some may be synonymous) in a single genus, Paradorydium. Collection records from grass, rushes, moss and Dracophyllum (Ericaceae) (Larivière et al. 2010) indicate that they live in habitats that are not recognised by G. ashmeadi to contain host eggs. The Iassinae are represented by two species of the worldwide genus Batracomorphus, both of which are also found in Australia and the Pacific Islands (Larivière et al. 2010). They have been collected from many types of plants, mostly such as Leptocarpus (Restionaceae), grasses and other grass-like habitats. They have also been collected from trees such as Hoheria and Plagianthus. The two species of Idiocerinae in New Zealand feed on Populus species (Salicaceae). Like other species in this subfamily that feed on poplars, they probably lay eggs singly under the soft bark tissue of new shoots. Other Idiocerinae from around the world also lay eggs within the plant tissue (e.g. Dietrich and McKamey 1990). The single macropsine species in New Zealand is the endemic Zelopsis nothofagi (Evans). It is found throughout much of the country and has been found predominantly on species of Nothofagus beech trees. Little is known of its biology, but other Macropsinae such as the six Oncopsis species that feed on trees in British woodlands, lay eggs singly into host plant tissues (Claridge and Reynolds 1972). The Tartessinae are represented by sixteen endemic species of Novothymbris spp. which have been collected from a variety of habitats from grasses and sedges through shrubs to trees (Larivière et al. 2010). Virtually nothing is known of their biology, but it is expected that they lay eggs singly in stems of host plants. Species of Typhlocybinae from around the world typically lay single eggs below the soft bark of young stem growth, or into the veins of leaves (e.g. Claridge and Wilson 1976). This is certainly the case for New Zealand’s exotic typhlocybines (pers. obs.), and there is no reason to think that the native species, for which the biology has not been studied, are any different.
Hence of the five species of Cicadellidae in New Zealand that may lay eggs sufficiently large to be considered hosts by G. ashmeadi, four can be rejected on biological grounds, either because they live and lay eggs in inappropriate habitats (both Paradorydium species and Paracephaleus hudsoni (Myers)), or because they lay single eggs (Rhytidodus decimaquartus (Schrank)). The remaining species is the adventive Euacanthella palustris, but even this is unlikely to be a host as it is most commonly found in grasslands, and probably lays eggs singly (Table 2). Finally, it may be ecologically significant that the Proconiini (and many Cicadellinae) are xylem feeders, whereas New Zealand’s Cicadellidae are all either phloem or mesophyll feeders.
Discussion
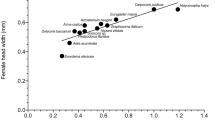
The absence from New Zealand of any leafhoppers within the same genus, tribe or subfamily (Cicadellinae) as GWSS, and the absence of any other close relatives of GWSS, means that none of the known hosts of G. ashmeadi occurs in New Zealand. The Cicadellidae that are present in New Zealand are all rather small insects. Nearly all of them are less than half the size of GWSS, and many are less than one-third the size. Host-testing experiments and field records in California have shown that G. ashmeadi does not attack eggs of small leafhoppers (<7 mm long), even if they are similar in disposition to those of GWSS. Hence, the risk to New Zealand’s leafhoppers from G. ashmeadi can be considered extremely low simply because they are too small. Even the largest cicadellid in New Zealand (the exotic Euacanthella palustris) is less than three quarters the size of GWSS. Extensive ecological studies have also been carried out on G. ashmeadi both in California and in countries where it has been released as a successful biocontrol agent against GWSS. These studies have shown that G. ashmeadi attacks only eggs in masses on the undersides of leaves of trees and bushes (not grasses). Hence, in addition to the size constraints, this combination of ecological features eliminates all New Zealand Cicadellidae as potential hosts of G. ashmeadi (Fig. 1).
Other ecological factors add to the confidence that New Zealand’s fauna are safe. The Proconiini (and some other Cicadellinae) ‘powder’ their egg masses using egg brochosomes, which are regularly structured, ultramicroscopic, proteinaceous particles synthesised within the Malpighian tubules. Most Cicadellidae produce brochosomes but relatively few use them to powder eggs (Rakitov 2004). The purpose of egg brochosomes is unknown, but it has been postulated that they may defend against parasitism or predation. It seems likely that G. ashmeadi associate egg brochosomes with host eggs, and may even use them as a prerequisite for initiating oviposition behaviour. However, no New Zealand species are known to use, or suspected of using, brochosomes to powder eggs.
Finally, the risk of displacement of other native insects by G. ashmeadi is also low. Gonatocerus is represented in New Zealand by about five undescribed species, belonging to the litoralis group (Noyes and Valentine 1989). This group, which may be adventive, probably attacks small leafhoppers that live on grasses and small perennial plants (Matthews 1986), and hence occupies different ecological habitats and niches from G. ashmeadi.
In conclusion, the risk to New Zealand’s fauna of non-target attack by G. ashmeadi can be considered extremely low. This conclusion is based on three key facts:
-
1.
The only known hosts of G. ashmeadi are absent from New Zealand.
-
2.
Seventy-four of the 79 species of New Zealand’s Cicadellidae and Membracidae are too small to be considered as hosts by G. ashmeadi (Fig. 1).
-
3.
None of the five largest species lays eggs in masses on the undersides of leaves of trees and bushes, and so none possesses the combination of ecological features of species recognised as hosts by G. ashmeadi (Fig. 1).
Faced with this risk profile, there is no scientific justification for conducting host-testing experiments in containment, because the environmental risks posed by G. ashmeadi in New Zealand are adequately assessed by reference to the published literature. The conclusion that host-testing is not scientifically justified does not imply that G. ashmeadi (or any other biocontrol agent with a demonstrably low risk profile) should immediately be allowed into New Zealand. Other issues, such as the potential economic and environmental benefits brought by the biocontrol agent, must also considered by the EPA (with the expectation that the benefits should outweigh the risks) before it is approved for release. Nevertheless, on the perhaps rare occasions that it can be shown that experimental host-testing in containment will not add useful data to the risk analysis, then a decision to release a biocontrol agent may conceivably be made very soon after the arrival of a new pest. Such a decision may potentially allow biocontrol to contribute in novel ways to an early pest management strategy, for example by helping to slow the spread of the new pest or even by aiding its eradication.
References
Berndt LA, Withers TM, Mansfield S, Hoare RJB (2009) Non-target species selection for host range testing of Cotesia urabae. N Z Plant Prot 62:168–173
Boyd EA, Hoddle MS (2007) Host specificity testing of Gonatocerus spp. egg-parasitoids used in a classical biological control program against Homalodisca vitripennis: a retrospective analysis for non-target impacts in Southern California. Biol Control 43:56–70
Charles JG, Dugdale JS (2011) Non-target species selection for host-range testing of Mastrus ridens. N Z Entomol 34:45–51
Claridge MF, Reynolds WJ (1972) Host plant specificity, oviposition behaviour and egg parasitism in some woodland leafhoppers of the genus Oncopsis (Hemiptera Homoptera: Cicadellidae). Trans Royal Entomol Soc Lond 124:149–166
Claridge MF, Wilson MR (1976) Diversity and distribution patterns of some mesophyll-feeding leafhoppers of temperate woodland canopy. Ecol Entomol 1:231–250
Dietrich CH, McKamey SH (1990) Three new idiocerine leafhopper (Homoptera: Cicadellidae) from Guyana with notes on ant-mutualism and subsociality. Proc Entomol Soc Wash 92:214–223
Dietrich CH, Rakitov RA, Holmes JL, Black WC IV (2001) Phylogeny of the major lineages of Membracoidea (Insecta: Hemiptera: Cicadomorpha) based on 28S rDNA sequences. Mol Phylogenet Evol 18(2):293–305
Fletcher MJ (2009) Identification keys and checklists for the leafhoppers, planthoppers and their relatives occurring in Australia and neighbouring areas (Hemiptera: Auchenorrhyncha). http://www1.dpi.nsw.gov.au/keys/leafhop/index.html. Accessed 27 Feb 2012
Grandgirard J, Hoddle MS, Petit JN, Percy DM, Roderick GK, Davies N (2007) Pre-introductory risk assessment studies of Gonatocerus ashmeadi (Hymenoptera: Mymaridae) for use as a classical biological control agent against Homalodisca vitripennis (Hemiptera: Cicadellidae) in the Society Islands of French Polynesia. BioControl Sci Techn 17:809–822
Grandgirard J, Hoddle MS, Petit JN, Roderick GK, Davies N (2008) Engineering an invasion: classical biological control of the glassy-winged sharpshooter, Homalodisca vitripennis, by the egg parasitoid Gonatocerus ashmeadi in Tahiti and Moorea, French Polynesia. Biol Invasions 10:135–148
Irvin NA, Hoddle MS (2006) The effect of intraspecific competition on progeny sex ratio in Gonatocerus spp. for Homalodisca coagulata egg masses: Economic implications for mass rearing and biological control. Biol Control 39:162–170
Knight WJ (1974) Leafhoppers of New Zealand: subfamilies Aphrodinae, Jassinae, Xestocephalinae, Idiocerinae, and Macropsinae (Homoptera: Cicadellidae) N Z. J Zool 1:475–493
Knight WJ (1987) Leafhoppers of the grass-feeding genus Balclutha (Homoptera, Cicadellidae) in the Pacific region. J Nat Hist 21:1173–1224
Kuhlmann U, Schaffner U, Mason PG (2006) Selection of non-target species for host specificity testing. In: Bigler F, Babendreier D, Kuhlmann U (eds) Environmental impact of invertebrates for biological control of arthropods: methods and risk assessment. CABI Publishing, UK, pp 15–37
Larivière M-C, Fletcher MJ, Larochelle A (2010) Auchenorrhyncha (Insecta: Hemiptera): catalogue. Fauna of New Zealand, vol 63. Manaaki Whenua Press, Lincoln, New Zealand, p 232
Matthews MJ (1986) The British species of Gonatocerus Nees (Hymenoptera, Mymaridae), egg parasitoids of Homoptera. Syst Entomol 11:213–229
Noyes JS (2011) Universal Chalcidoidea Database. World Wide Web electronic publication. http://www.nhm.ac.uk/chalcidoids. Accessed 27 Feb 2012
Noyes JS, Valentine EW (1989) Mymaridae (Insecta:Hymenoptera): introduction and review of genera. Fauna of New Zealand, vol 17. DSIR Publishing, Wellington, New Zealand, p 95
Petit JN, Hoddle MS, Grandgirard J, Roderick GK, Davies N (2008) Short-distance dispersal behaviour and establishment of the parasitoid Gonatocerus ashmeadi (Hymenoptera: Mymaridae) in Tahiti: implications for its use as a biological control agent against Homalodisca vitripennis (Hemiptera: Cicadellidae). Biol Control 45:344–352
Rakitov RA (2004) Powdering of egg nests with brochosomes and related sexual dimorphism in leafhoppers (Hemiptera: Cicadellidae). Zool J Linn Soc 140:353–381
Turner WF, Pollard HN (1959) Life-histories and behavior of five insect vectors of phony peach disease. U S Dep Agric Technic Bull, USA 1188:1–32
van Driesche RG, Reardon R (2004) Assessing host ranges for parasitoids and predators used for classical biological control: a guide to best practice. Forest Health Technology Enterprise Team, USDA, Morgantown, USA
van Lenteren JC, Cock MJW, Hoffmeister TS, Sands DPA (2006) Host specificity in arthropod biological control, methods for testing and interpretation of the data. In: Bigler F, Babendreier D, Kuhlmann U (eds) Environmental impact of invertebrates for biological control of arthropods. Methods and risk assessment. CABI Publishing, UK, pp 38–63
Vickerman DB, Hoddle MS, Triapitsyn S, Stouthamer R (2004) Species identity of geographically distinct populations of the glassy-winged sharpshooter parasitoid Gonatocerus ashmeadi: morphology, DNA sequences, and reproductive compatibility. Biol Control 31:338–345
Wells JM, Raju BC, Hung HY, Weisburg WG, Mandelco-Paul L, Brenner DJ (1987) Xylella fastidiosa gen. nov., sp. nov.: gram-negative, xylem-limited, fastidious plant bacteria related to Xanthomonas spp. Int J Syst Bacteriol 37:136–143
Zahniser JN, Dietrich CH (2010) Phylogeny of the leafhopper subfamily Deltocephalinae (Hemiptera: Cicadellidae) based on molecular and morphological data with a revised family-group classification. Syst Entomol 35:489–511
Acknowledgments
This paper is based upon a report to New Zealand’s Sustainable Farming Fund. New Zealand Winegrowers and New Zealand Citrus Growers Inc. supported this research, which was finalised with funding from New Zealand’s ‘Science Solutions for Better Border Biosecurity (B3) programme’ http://b3nz.org/. Fig. 1 was provided by David Logan, The New Zealand Institute for Plant & Food Research Limited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Stefano Colazza
Rights and permissions
About this article
Cite this article
Charles, J.G. Assessing the non-target impacts of classical biological control agents: is host-testing always necessary?. BioControl 57, 619–626 (2012). https://doi.org/10.1007/s10526-012-9449-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-012-9449-z