Abstract
In January 2002, the first biological control program was implemented on the Galapagos Islands with the release of the Australian coccinellid Rodolia cardinalis Mulsant to control the invasive cottony cushion scale, Icerya purchasi Maskell. This was the first time that Galapagos authorities had approved the introduction of a biological control agent to this iconic archipelago and, because of this precedent, it was important to monitor and evaluate its behaviour soon after its introduction. Surveys were carried out after the release of R. cardinalis in 2002 to confirm establishment on Santa Cruz Island. In 2009, seven years post-release, a broader survey was done to document spread throughout the archipelago. Directly after the release of R. cardinalis in 2002, a predator exclusion study and field observations were carried out on scale insect populations on white mangrove (Laguncularia racemosa [L.] Gaertn. F.) on Santa Cruz Island to document impact. In less than three months after R. cardinalis was released in 2002, populations of I. purchasi on white mangrove that were exposed to the predator in the exclusion experiment, or were monitored in the field, had declined by 99–100%. Results suggest that R. cardinalis played a key role in this decline, possibly in combination with high rainfall. Rodolia cardinalis dispersed quickly after its release and by 2009 was found in a wide variety of habitats on seven of the eight islands surveyed that had records of I. purchasi. Two of these were self-introductions. Further monitoring is recommended to determine whether this biological control agent has successfully reduced scale insect numbers on other valued plant species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impact studies following the release of a biological control agent are a necessary step for classical biological control programs (Gurr and Wratten 2000; Lynch et al. 2001; Stanley and Julien 1998). These types of studies are important because they evaluate the efficacy of the agent in controlling the target pest and validate the predictions made in the pre-release screening trials concerning non-target impacts (Barton et al. 2007; Blossey 1999; Dudley and Kazmer 2005). Post-introduction evaluations also provide important feedback for biological control practitioners for improving techniques to assess the impact of released biological control agents. Additionally, positive outcomes that are observed from post-introduction evaluations can help improve the reputation of classical biological control, thereby promoting consideration of its use for invasive species suppression by decision makers and the community as a whole.
Post-release evaluations are critical when classical biological control is used against an environmental pest in ecosystems with endemic flora and fauna that have small populations because impacts, both potentially beneficial and negative, need to be determined to assess program success. Post-release information was politically and administratively important in the conduct of the first government-sanctioned biological control program carried out in the Galapagos Islands in 2002, the release of Rodolia cardinalis Mulsant (Coleoptera: Coccinellidae) to control the invasive cottony cushion scale, Icerya purchasi Maskell (Hemiptera: Monophlebidae) (Causton et al. 2006; Causton 2009). Reporting on the initial results of the biological control program was not only required by scientists and Galapagos National Park managers, some of whom were hesitant about using biological control in Galapagos, but of interest to the public, many of whom were aware of this project. Extensive public awareness campaigns were carried out before the program and these continued once it had started, with many members of the community being actively involved in releasing adult R. cardinalis and monitoring its establishment. Rapid and positive results were expected, and this was anticipated as likely to quell any concerns that might arise that natural enemy introductions could cause more environmental problems. These efforts also provided an opportunity for the Galapagos National Park Service (GNPS) to recognize the efforts of the community helpers who helped mitigate the impacts of this invasive species.
The flora of the Galapagos was seriously affected by the invasion of I. purchasi. This scale insect was first reported in the archipelago in 1982 and by 1996 it had spread to 15 of the 18 larger islands. By 2002, it was known that I. purchasi was feeding on 80 plant species on the islands: 31 endemic, 31 native but not endemic, and 18 introduced (Causton 2001; Causton et al. 2006). Plant mortality caused by cottony cushion scale caused significant population declines and changes in the International Union for Conservation of Nature (IUCN) threat status for some threatened, endemic species such as Darwiniothamnus tenuifolius (Hook, f.) on Alcedo volcano on the island of Isabela (Causton 2001). Furthermore, three endemic moths that are specialist feeders on D. tenuifolious disappeared from the area where host plant populations were most severely damaged by I. purchasi (Roque-Albelo 2003). Coastal mangrove ecosystems were also damaged by I. purchasi, in particular the white mangrove, Laguncularia racemosa (L.) Gaertn. F. Studies in Galapagos with young, potted white mangrove plants demonstrated that feeding by I. purchasi significantly reduced branch production and growth, as well as reducing root growth (Causton 2001). Mangroves occur on the coastlines of most islands in the Galapagos and are considered a key engineering species providing food and refuge for marine and littoral species. The white mangrove is also an important nesting area for the critically endangered mangrove finch, Camarhynchus heliobates Gould, a species with only about 110 individuals restricted to small pockets of mangroves on the western coast of Isabela Island (Fessl et al. 2010).
A review by a multi-agency team of the effects of the I. purchasi invasion and the available control options concluded that classical biological control using R. cardinalis was the only option available for effectively reducing the impact of I. purchasi in the Galapagos Islands. This natural enemy was chosen because it had controlled I. purchasi in a wide range of climatic conditions in many countries and the majority of available evidence suggested that it also had a very restricted prey range indicating it probably would not threaten native and endemic insects in the Galapagos Islands (Causton et al. 2004). Extensive trials were conducted by scientists at the Charles Darwin Research Station (CDRS) to confirm the impact of the target pest, I. purchasi, on native plants and on the safety of R. cardinalis to native invertebrates and vertebrates (Causton 2003; Causton et al. 2004; Lincango et al. 2011). After reviewing a risk analysis produced by CDRS, permission was granted by the GNPS for the release of R. cardinalis from quarantine. Priority areas for introductions were identified based on the location of threatened and ecologically important plant species. Here we report on the establishment and impact of R. cardinalis soon after its release in 2002 and again in 2009, seven years after the initiation of releases of R. cardinalis. The studies in 2002 were conducted on Santa Cruz Island and focused primarily on the effect of R. cardinalis on suppressing I. purchasi on two heavily infested stands of white mangrove. Data from 2009 reported here concern further distributional records for R. cardinalis in urban, agricultural, and protected areas throughout the archipelago.
Methods
Between January 2002 and January 2003, 1709 R. cardinalis adults, 27 pupae, and five larvae were released on Fernandina, Floreana, Genovesa, Isabela, Marchena, Pinta, Pinzon, Rabida, San Cristobal, Santa Cruz, and Santiago Islands (Table 1). Additional releases (total 497 adults) were made on Isabela, Marchena, Pinta, Santa Cruz, and San Cristobal in 2003–2005 (Table 1). This was either because R. cardinalis was not thought to have established (e.g., Marchena and Pinta) or because members of the public requested additional beetles (Isabela, Santa Cruz, and San Cristobal). All beetles originated from a colony that had been maintained at a quarantine facility at CDRS on Santa Cruz Island since 1999. The source population for this colony was obtained from a laboratory colony at CSIRO, Brisbane, Australia.
At the study sites on Santa Cruz Island, 380 adult beetles were released between 25 and 31 January 2002 (Fig. 1a). A total of 100 adults were liberated on infested white mangroves along the coast (at Hotel Galapagos and GNPS headquarters) and 80 were deployed on a heavily infested mango tree in the downtown Puerto Ayora (Fig. 1a). In addition to this, 200 R. cardinalis adults were released on white mangrove stands at Punta Estrada, across the bay and one mile from Puerto Ayora (Fig. 1a).
Establishment and spread of R. cardinalis
Ten weeks following the release of R. cardinalis, all known host plants of I. purchasi along the perimeter of the town of Puerto Ayora were surveyed for R. cardinalis (Fig. 1a). The presence of any developmental stage of R. cardinalis was noted and the location of each plant was recorded with a handheld GPS. Between 2002–2009, known host plants of I. purchasi (Table 2) were surveyed for R. cardinalis at additional locations on Santa Cruz and on other islands in the archipelago.
In 2009, a two year monitoring program was initiated and between October and December 2009, Baltra, Champion, Española, Fernandina, Floreana, Isabela, Marchena, San Cristobal, and Santa Cruz Islands were surveyed for the presence of I. purchasi and R. cardinalis via visual observations of native plants or with yellow sticky traps hung in bushes and trees. Sticky traps were placed in National Park areas on each island as well as in urban and agricultural areas on the inhabited islands (Baltra, Floreana, Isabela, San Cristobal, Santa Cruz). Up to thirty traps were placed on each island and traps were deployed for one–two weeks to trap adult R. cardinalis.
Effect of R. cardinalis on population numbers of I. purchasi
Two studies were conducted between January and April 2002 at R. cardinalis release sites on Santa Cruz Island: 1) exclusion cages were used to measure the effects of the predator at Punta Estrada and 2) unmanipulated populations of I. purchasi and R. cardinalis were monitored on marked branches of heavily infested stands of L. racemosa along the coast at Puerto Ayora (Hotel Galapagos to GNPS) (Fig. 1a).
Predator exclusion studies
Two experimental conditions were established: (1) controls that were I. purchasi-infested branches enclosed in sleeve cages to prevent access by R. cardinalis (following Luck et al. 1998; Prasad 1989; van Driesche and Bellows 1996) and (2) treatment branches that were continuously exposed to foraging R. cardinalis. A preliminary study was conducted to determine the minimum sample size required to estimate scale density. To do that, mangrove plants were observed from a distance of 1 m through a transparent plastic sheet that was divided into numbered squares. Branches falling into 21 randomly chosen squares in the viewing sheet were identified and the adult females of I. purchasi on each chosen branch were counted. Data was plotted and an average sample number curve (ASN) (Southwood 1978) used to determine the minimum sample size, which was determined to be ten branches (i.e., ten replicates). To compensate for any unexpected damage or branch mortality that might occur during the experiment, we sampled 15 branches/treatment. Thirty branches were randomly selected using the methodology described for the preliminary study and randomly assigned to the experimental treatments. Only branches with more than ten I. purchasi were selected. During the experiment, five cages were damaged and these were eliminated from the experiment leaving ten caged branches, and 15 uncaged branches.
The first 50 cm of each branch, the part of the branch where most scale insects are typically located (Prasad 1992), was marked. Cylindrical cages (50 cm in diameter and 80 cm in length) were used to cover the control branches. The wire cylinders were covered with muslin, which was supported by wire rings at each end of the cage. Wire supports prevented contact between the muslin and the branch, which was intended to increase air circulation and reduce honeydew contamination and excessive moisture (van Driesche and Bellows 1996). Sleeve cages were closed and sealed on the trunk side to prevent the entry or exit of any insects. Neighbouring branches were moved away from the cages to prevent branches acting as bridges for natural enemies and other insects thus minimizing the likelihood of their unwanted entry. In addition, sticky traps were also placed around cages to further reduce the possibility of invasion of control cages by R. cardinalis or other predators.
Scale density was measured on each branch on six relatively equally spaced dates between 22 January 2002 and 19 April 2002. The first count was made three days before the release of R. cardinalis in the immediate vicinity of the experimental set up. The second count was made one month later, and subsequent counts were taken every 14 or 15 days. Scale insect life stages (instars 1, 2, 3, and adult females) were recorded separately during counts. Scale insects on branches were counted from the furthest growing tip, working backwards to the trunk end of the branch along a 50 cm section. Rodolia cardinalis adults, pupae and larvae were also recorded. The presence of pupal exuvia and eggs was noted, but these were not included in the calculations of population density because eggs are hard to detect without dissection (they are hidden under or inside I. purchasi) and pupal exuviae remain attached to plants for an extended period of time. On each sampling date, caged branches were checked for the presence of any unwanted insects or spiders, and these were removed if found.
Impact of R. cardinalis under unmanipulated field conditions
White mangrove trees along the coast of Puerto Ayora from Hotel Galapagos to the GNPS dock were used in this study (Fig. 1a). The methodology for selecting the branches was similar to the predator exclusion experiment described above. Twenty-four branches were randomly selected, and the first 50 cm of the branch was marked for monitoring the density of I. purchasi in the presence of the released population of R. cardinalis. Adult females of I. purchasi and adults, pupae and larvae of R. cardinalis were counted on each branch at six relatively equally spaced times between 15 January 2002 and 18 April 2002. The first counts were taken ten days before the release of R. cardinalis in the neighbouring area. The second count was made five weeks later, with subsequent counts being made every 12–15 days.
Data analysis
For the predator exclusion studies, means and standard deviations were calculated for each developmental stage of I. purchasi and for all life stages combined for each experimental treatment at each time point. There were no missing observations. Mixed models (Littell et al. 2006) were used to compare differences between experimental treatments, including time effects and treatment × time interactions. The model for the mean total count, \( Y_{ijtk} \), of branch j at time t on occasion k under experimental condition i was the following:
where i represents treatment, j branch, t time (day), and k occasion of measurement.
In this model, \( \mu + \alpha_{i} \) represents the average per branch for each scale insect life stage or for all life stages combined over the time point for each experimental treatment, i, \( \tau_{i} + \alpha \tau_{it} \) represents the deviation from the average at time t under experimental treatment i, and B ij represents the deviation for the jth randomly selected branch under experimental treatment i, where we assume that \( E\left( {B_{ij} } \right) = 0 \) and \( \text{var} (B_{ij} ) = \sigma^{2} \). The final term, E ijtk , represents the residual variability including random replication error for the kth replication (where k = 1) of the count on the same branch, and at the same time and under the same experimental treatment, and variation due to possible heterogeneity of fixed effects between branches where \( E(E_{ijtk} ) = 0 \) and \( \text{var} (E_{ijtk} ) = \sigma_{e}^{2} \). This model assumes that branches are randomly assigned to treatments, with σ2 representing the variance in the average (over time) branch effect. The model includes fixed effects representing a main effect for each treatment (an average treatment effect), \( \alpha_{i} \); a time effect, \( \tau_{t} \); and a treatment × time interaction, \( \alpha \tau_{it} \). Random effects include the branch effect, and the residual error.
We also represented parameters for the means of each life stage and all life stages combined over time by slope over intercept. We fit a linear regression model to time (using day from introduction of R. cardinalis) as a time scale, and allowed slopes and intercepts to vary between treatments using the no cage treatment as a reference group and the change in slope (increment) as the estimate for the closed cage treatment. Data more than 56 days after the release of R. cardinalis were excluded from this analysis because of low scale insect density on uncaged branches.
Results
Establishment and spread of R. cardinalis
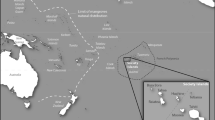
Surveys demonstrated that R. cardinalis quickly became established at release sites in Puerto Ayora, Santa Cruz Island. Ten weeks after it was first released, R. cardinalis was present on 82% (n = 60) of plants infested with I. purchasi that were surveyed along the perimeter of Puerto Ayora. Immature and adult R. cardinalis were recorded on 11 plant species from ten families (Table 2). Furthermore, R. cardinalis spread quickly after its release to other parts of Santa Cruz Island. Eight weeks after release (March 2002), larvae and pupae were found on Scaevola plumieri (L.) plants along the coast at Tortuga Bay, three km from the release site (Fig. 1b). Twenty-two weeks after it was released in Puerto Ayora, R. cardinalis was detected on the north side of Santa Cruz Island (45 km). At the same time, adult R. cardinalis were also found on Baltra Island, which is separated from the northern coast of Santa Cruz by an ocean channel approximately 200 m wide (Fig. 1b). A 2003 survey found that R. cardinalis had dispersed naturally to North Seymour Island, north of Baltra (Fig. 1b).
During an archipelago-wide survey in 2009, R. cardinalis was found on all of the islands that were surveyed (Baltra, Champion, Fernandina, Floreana, Isabela, Marchena, San Cristobal, Santa Cruz) with the exception of Pinzon, which was only partially surveyed, and Española, where there were no records of I. purchasi (Fig. 2). Rodolia cardinalis was observed or collected on sticky traps in a wide range of vegetation types, ranging from xeric habitats to humid highlands, and in dry, high altitude zones on the rims of volcanoes (altitudinal range of 0 to ~1200 m elevation), as well as in urban, agricultural, and National Park areas.
Between 2002 and 2009, R. cardinalis was recorded on 40 of 112 (36%) of the known host plants of I. purchasi (Table 2). Most of these records were reported by CDRS scientists and GNPS personnel during field trips undertaken for other projects, and not from surveys specifically designed to study the distribution of R. cardinalis. Ten of these plant records are endemic plant species and include the threatened species D. tenuifolius, Calandrinia galapagosa H. St. John, and Scalesia cordata A. Stewart. Rodolia cardinalis has also been found on 16 native but not endemic species, including all four of the mangrove species native to the Galapagos Islands (Avicennia germinans L., Conocarpus erectus L., L. racemosa, and Rhizophora mangle L.), and 14 introduced plant species.
Effect of R. cardinalis on population numbers of I. purchasi
Predator exclusion studies
Overall, a decrease in the number of I. purchasi over time was observed in both experimental treatments. However, populations that were exposed to the predator, R. cardinalis, declined at a faster rate than populations that were isolated from the predator in cages (Table 3; Fig. 3).
One month after the release of R. cardinalis, population numbers of I. purchasi on uncaged branches had declined by 46% from an average of 120.5 ± 68 to 64.9 ± 43.2 individuals per branch (n = 15) and continued to decline, reaching zero 85 days after R. cardinalis was released. With the exception of some recruitment in the first and second instars (between 29 and 42 days post-release of R. cardinalis), all developmental stages declined at each sampling date (Table 3). In contrast, in the exclusion cages, first instars and adult I. purchasi increased during the first month (January 22–February 21, 2002), causing a 9% increase in total population numbers from an average of 176.7 ± 91 to 192. 8 ± 130 individuals per branch (n = 10). Following this, population numbers on caged branches declined although recruitment was observed in the immature stages (1–3 instars) during the second month of the experiment (Table 3). In both the treatment and control, 28 days after the release of R. cardinalis, more than a half of the I. purchasi population was composed of adult females. This ratio was maintained in the caged branches for at least another month. In contrast, on uncaged branches, the mean proportion of I. purchasi adults declined steadily, reaching zero 70 days after the release of R. cardinalis (Table 3).
Rodolia cardinalis was present on 73% of uncaged branches one month after it was released with an average of 1.9 ± 2 R. cardinalis individuals per branch (n = 15) (Fig. 3). All immature stages of R. cardinalis were observed, demonstrating predator reproduction on the scale insect colonies. Predator numbers peaked 42 days after R. cardinalis was released with a mean of 2.8 ± 3.8 individuals per branch, after which numbers declined, reaching zero 70 days after release. Caged branches were not invaded by the predator at any time during the experiment.
A significant difference in mean counts per branch between experimental treatments was found for all life stages of I. purchasi (range in F values: 4.1–8.1, df = 1, 115, P ≤ 0.05 for 1–3 instars, F = 22.6, df = 1, 115, P < 0.0001 for adults, and F = 13.96, df = 1, 115, P < 0.0003 for all life stages of I. purchasi combined) with consistently higher I. purchasi counts in the exclusion cages (Tables 3 and 4). It is important to note that there was significant variability (approximately one fifth of the residual variation) in I. purchasi counts between branches for all life stages. Scale insect populations at all life stages declined in both treatments over time (range in F values: 5.4–33.7; df = 5, 115; P ≤ 0.0002); however, for all life stages there was evidence of an experimental treatment × time interaction, indicating that the time effect was different between experimental treatments. The P-values for this interaction were less than 0.25 for 1–3 instars and significantly different for adult females (F = 3.89; df = 5, 115; P = 0.003) and when all life stages were combined (F = 2.52; df = 5, 115; P = 0.03) (Table 4). When slopes were estimated for I. purchasi counts for treatment and control groups, over the period up to 56 days after the release of R. cardinalis, a population decrease of 1.7 (SE = 0.47) scale insects per day was observed in uncaged branches compared to 1.1 (SE = 0.87) among caged branches, indicating that I. purchasi declined at a faster rate when it was exposed to the predator, R. cardinalis (Table 5). A steeper negative slope was evident for the uncaged branches for all developmental stages (except for adult females) demonstrating a faster rate of population decrease when scale insects were exposed to predators, but this was not statistically significant at the 0.05 level.
Impact of R. cardinalis under unmanipulated field conditions
On mangrove branches where adult female I. purchasi densities were followed after predator release, without the use of cages, I. purchasi numbers showed an initial increase but then after day 25 post-release, declined steadily, reaching almost zero at day 83 (a 99.8% reduction in two months) (Fig. 4). Twenty five days after the release of the predator, I. purchasi numbers had increased by 35% to a mean density of 61.9 ± 38.6 adult females per branch (n = 24), with up to 168 adult females found on one branch. At this time, a mean of 1.2 ± 3.7 R. cardinalis per branch were observed. Thirty-nine days after the biological control program was initiated, I. purchasi populations had declined to an average of 54.4 ± 37.6 adult females per branch and continued to decline, with only two adult females left 83 days after release. Predator numbers peaked 39 days after R. cardinalis was released with a mean of 2.2 ± 3.9 individuals per branch, following which numbers declined, with only one individual counted at 83 days post-release (Fig. 4).
Discussion
The coccinellid R. cardinalis, released for the classical biological control of I. purchasi in the Galapagos Islands in 2002, has established and dispersed throughout the archipelago. Furthermore, it has adapted to a wide range of habitats and has been shown experimentally to be at least partially responsible for reducing I. purchasi populations on one important native plant (white mangrove).
Rodolia cardinalis has established successfully in most parts of the archipelago despite the release of relatively low numbers (2,206 individuals). This is particularly notable for Fernandina Island, a semi-pristine island of 248 mi2 where populations of R. cardinalis originated from just 26 individuals. Rodolia cardinalis has also proven to be a good intra- and inter-island disperser. Island-wide surveys indicate that R. cardinalis is now widely present in many areas and habitats (including urban, agricultural, and natural areas). It has also been found preying on I. purchasi on islands where it was not originally released (i.e., Baltra, North Seymour, and Champion Island). Furthermore, our studies on Santa Cruz Island demonstrated that it dispersed quickly after its release and exhibited a high searching capacity, similar to that observed in its native range (Australia), where it was able to detect and destroy cottony cushion scales located 500 m from other I. purchasi colonies within a week (Prasad 1990).
Reproducing populations of R. cardinalis were observed on I. purchasi at two study sites on Santa Cruz Island within a month of the beetle being released. At Punta Estrada (site of the exclusion study) and on the coast of Puerto Ayora, I. purchasi populations that were exposed to the predator declined by 99–100% within three months of R. cardinalis release. Within a month after the release of its natural enemy, I. purchasi populations had been almost halved at Punta Estrada, the site with the highest number of beetles (200) released. At both study sites, the trends of I. purchasi and R. cardinalis followed the classical predator–prey response curve with the predator increasing in response to prey densities and then declining once pest populations diminished locally (van Driesche et al. 2008). The rapid drop in scale insect numbers, suggests that the predator had an impact on I. purchasi populations. Nevertheless, it was hard to isolate the effects of the predator from other factors because survivorship also decreased in populations of I. purchasi that were protected from the predator by cages. When I. purchasi populations are protected from natural enemy attacks because of exclusion cages, populations should become significantly higher in comparison to populations that are not protected from foraging natural enemies (Prasad 1989).
Cages in our experiment did initially show increased I. purchasi population growth, but after one month numbers started to decline. A deterioration in the cage environment may have been responsible for scale insect mortality. The exclusion study was conducted in the hot, rainy season, and 2002, the year of the experiment, was a particularly wet year because of an el Niño event. High rainfall and high temperatures are likely to have increased humidity within the cages, which in turn may have promoted the growth of pathogens: an unidentified white fungus was found on many I. purchasi towards the end of the experiment. Between January and April, 459 mm of rainfall were recorded with more than 75% falling in the last two months of the trial. The months of highest rainfall were also associated with the highest temperatures of the year (CDF 2011). Previous collection records found that lower population numbers of I. purchasi were recorded between January and May (Causton 2001; Roque-Albelo and Causton 1999), suggesting that I. purchasi does not thrive in periods of hot, wet weather.
A marked difference in the rate of decline between I. purchasi populations that were exposed to the predator (uncaged) and populations that were protected from the predator (caged) does, however, suggest a predator effect. Although, initial total population counts for I. purchasi were similar for both experimental treatments, the rate of decline of I. purchasi over the 12 week experiment was greater on branches that were exposed to R. cardinalis. Furthermore, additional recruitment was prevented because the proportion of reproducing adults in the uncaged population declined quickly.
The results from the exclusion experiment on white mangrove, though not conclusive, suggest that R. cardinalis played a key role in reducing I. purchasi populations on this ecologically important species. It is possible that high rainfall and temperature also contributed to this decline. Mangrove forests are important refuges for littoral and terrestrial fauna, as well as important nesting areas for marine and terrestrial birds. Within a year, substantial recovery of white mangroves was observed along the coast of Puerto Ayora and at Punta Estrada. Highly stressed mangroves that had been covered with I. purchasi and a black sooty mold that grows on the honeydew excreted by the cottony cushion scale, had returned to a lush green colour and new growth was notable (see van Driesche et al. 2010). Such fast recuperation was not only encouraging to conservation managers, but permitted the local community to see these changes and experience first-hand the results of conservation science in action. This was especially important because until then most of the large conservation projects in the Galapagos had been conducted on uninhabited islands and some residents expressed the feeling that nothing was ever done to help local communities.
From a conservation management perspective, R. cardinalis has demonstrated characteristics of an effective natural enemy and should be highly beneficial for the archipelago’s natural ecosystems. Self-dispersal mechanisms have enabled R. cardinalis to track I. purchasi into ecologically sensitive areas that are remote and difficult to reach, making for a highly cost effective management program. The presence of R. cardinalis on a wide range of plant species damaged by I. purchasi suggests that the predator is mitigating the impact of this invasive species on other valued plant species. Continuing and future surveys of these affected species will over time enlarge the record of R. cardinalis’ benefits.
References
Barton J, Fowler SV, Gianotti AF, Winks CJ (2007) Successful biological control of mist flower (Ageratina riparia) in New Zealand: agent establishment, impact and benefits to the native flora. Biol Control 40:370–385
Blossey B (1999) Before, during and after: the need for long-term monitoring in invasive plant species management. Biol Invasions 1:301–311
Causton CE (2001) Dossier on Rodolia cardinalis Mulsant (Coccinellidae: Cocinellinae) a potential biological control agent for the cottony cushion scale Icerya purchasi Maskell (Margarodidae). Charles Darwin Foundation, Galapagos, Unpublished report
Causton CE (2003) Ensuring compatibility of biological control of Icerya purchasi Maskell with conservation in Galapagos: development of procedure to evaluate risk. In: van Driesche RG (ed) Proceedings of the First International Symposium for the Biological Control of Arthropods, HI. FHTET-03–05. USDA-FS, Morgantown, USA, pp 448–457
Causton CE (2009) Success in biological control: the scale and the ladybird. In: DeRoy T (ed) Preserving Darwin’s legacy, Galapagos, pp 184–190
Causton CE, Lincango MP, Poulsom TGA (2004) Feeding range studies of Rodolia cardinalis (Mulsant), candidate biological control agent of Icerya purchasi Maskell in the Galápagos islands. Biol Control 29(3):315–325
Causton CE, Peck SB, Sinclair BJ, Roque-Albelo L, Hodgson CJ, Landry B (2006) Alien insects: threats and implications for the conservation of the Galápagos Islands. Ann Entomol Soc Am 99:121–143
CDF (2011) Charles Darwin Foundation Climate Database. http://www.darwinfoundation.org/datazone/climate/. Cited October 2011
Dudley TL, Kazmer DJ (2005) Field assessment of the risk posed by Diorhabda elongata a biocontrol agent for control of saltcedar (Tamarix spp.) to a nontarget plant, Frankenia salina. Biol Control 35:265–275
Fessl B, Young HG, Young RP, Rodríguez-Matamoros J, Dvorak M, Tebbich S, Fa JE (2010) How to save the rarest Darwin’s finch from extinction: the mangrove finch on Isabela Island. Philos Trans R Soc B 365:1019–1030
Gurr G, Wratten S (2000) Biological control: measures of success. CABI, Wallingford, UK
Lincango M, Hodgson C, Causton CE, Miller D (2010) An updated checklist of scale insects (Hemiptera: Coccoidea) of the Galapagos Islands, Ecuador. Galapagos Res 67:3–7
Lincango M, Causton CE, Calderon Alvarez C, Jiménez-Uzcátegui G (2011) Evaluating the safety of Rodolia cardinalis to two species of Galapagos finch; Camarhynchus parvulus and Geospiza fuliginosa. Biol Control 56:145–149
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for Mixed Models, 6th edn. SAS Institute Inc., Cary
Luck RF, Shepard BM, Kenmore PE (1998) Experimental methods for evaluating arthropod natural enemies. Ann Rev Entomol 33:367–391
Lynch LD, Hokkanen HM, Babendreier D, Bigler F, Burgio G, Gao Z-H, Kuscke S, Loomans A, Menzler-Hokkanen I, Thomas MB, Tommasini G, Waage JK, van Lenteren JC, Zeng Q–Q (2001) Insect biological control and non-target effects: a European perspective. In: Wajnberg E, Scott JK, Quimby PC (eds) Evaluating indirect ecological effects of biological control. CABI Publishing, Wallingford, UK, pp 99–125
Prasad YK (1989) The role of natural enemies in controlling Icerya purchasi in South Australia. Entomophaga 34:391–395
Prasad YK (1990) Discovery of isolated patches of lcerya purchasi by Rodolia cardinalis: a field study. Entomophaga 35:421–429
Prasad YK (1992) Observations on the seasonal abundance of Icerya purchasi on Acacia baileyana in Adelaide, South Australia. BioControl 37:115–121
Roque-Albelo L (2003) Population decline of Galapagos endemic Lepidoptera on Volcan Alcedo (Isabela island, Galapagos Islands, Ecuador): an effect of the introduction of the cottony cushion scale? Bull Inst R Sci Nat Belg Entomol 73:1–4
Roque-Albelo L, Causton CE (1999) “El Niño” and introduced insects in the Galapagos Islands: different dispersal strategies, similar effects. Noticias de Galapagos 60:30–36
Southwood TRE (1978) Ecological methods, 2nd edn. Chapman and Hall, London, UK
Stanley JN, Julien MH (1998) The need for post-release studies to improve risk assessments and decision making in classical biological control. In: Zalucki M, Drew R, White G (eds) Pest management-future challenges: Proceedings of the 6th Australasian Applied Entomological Research Conference. The Cooperative Research Centre for Tropical Pest Management, Australia, pp 561–564
van Driesche RG, Bellows TS Jr (1996) Biological control. Chapman and Hall, New York, USA
van Driesche RG, Hoddle MS, Center T (2008) Control of pests and weeds by natural enemies: an introduction to biological control. Wiley, New York, USA
van Driesche RG, Carruthers RI, Center T, Hoddle MS, Hough-Goldstein J, Morin L, Smith L, Wagner D, Blossey B, Brancatini V, Casagrande R, Causton CE, Coetzee JA, Cuda J, Ding J, Fowler SV, Frank JH, Fuester R, Goolsby J, Grodowitz M, Heard TA, Hill MP, Hoffmann J, Huber J, Julien M, Kario MTK, Kenis M, Mason P, Medal J, Messing R, Miller R, Moore A, Neuenschwander P, Newman R, Norambuena H, Palmer WA, Pemberton R, Perez Panduro A, Pratt S, Rayamajhi M, Salom S, Sands D, Schooler S, Sheppard A, Shaw R, Schwarzländer M, Tipping PW, van Klinken R (2010) Classical biological control for the protection of natural ecosystems: past achievements and current efforts. Biol Control 54(1):S2–S33
Acknowledgments
We are very grateful to the GNPS park guards, V. Carrion, H. Herrera, N. d’Ozouville, G. Merlen, L. Roque-Albelo, N. Chasquilin, C. Sevilla, P. Lincango, J. Loayza, H. Rogg, T. Poulsom, F. Bersosa, R. Azuero, A. Mieles and C. Crespo for help with the survey work. We thank Sonia Cisneros (CDF) for all the logistical help, the Galápagos National Park Service for allowing insect sampling in the Park, Agrocalidad-SICGAL for transport and advice, and TAME for reduced airfares. CCA would also like to thank Alberto Ramirez, Pontificia Universidad Javeriana, Colombia for invaluable input to her thesis. This publication is contribution number 2045 of the Charles Darwin Foundation for the Galapagos Islands.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Kevin Heinz
Rights and permissions
About this article
Cite this article
Calderón Alvarez, C., Causton, C.E., Hoddle, M.S. et al. Monitoring the effects of Rodolia cardinalis on Icerya purchasi populations on the Galapagos Islands. BioControl 57, 167–179 (2012). https://doi.org/10.1007/s10526-011-9429-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-011-9429-8