Abstract
Brazilian peppertree, Schinus terebinthifolius Raddi (Sapindales: Anacardiaceae), is a highly successful invasive species in the continental United States, Hawaiian archipelago, several Caribbean Islands, Australia, Bermuda, and a number of other countries worldwide. It also is one of only a few invasive intraspecific hybrids that has been well characterized genetically. The natural enemy complex of Brazilian peppertree includes two thrips and two psyllids that appear to be highly adapted to specific haplotypes or their hybrids. Successful biological control of Brazilian peppertree will require careful matching of the appropriate natural enemies with their host plant genotypes. The Brazilian peppertree model reviewed here could provide a useful framework for studying biological control agents on other invasive weed species that have exhibited intraspecific hybridization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-native plants often become invasive when introduced outside their native ranges (Pimentel 2002). There are a number of hypotheses that have been proposed to explain invasion success including preadaptation (Baker 1965; Parker et al. 2003; Richards et al. 2006), escape from natural enemies (Williams 1954; Keane and Crawley 2002), propagule pressure (Williamson 1996), empty niches (Elton 1958; MacArthur 1970), invasional meltdown (Simberloff and Von Holle 1999), evolution of increased competitive ability (Blossey and Nötzold 1995), novel weapons (Callaway and Ridenour 2004), and diversity-invisibility (Elton 1958). Recently, there is an increasing emphasis on post-introduction evolution as an important determinant of invasion success (Sakai et al. 2001; Lee 2002; Cox 2004; Prentis et al. 2008; Suarez and Tsutsui 2008). There is accumulating evidence that invasive plants can undergo rapid adaptive evolution in their new range including the evolution of latitudinal or altitudinal clines (Maron et al. 2004, 2007; Keller et al. 2009), increased phenotypic plasticity (Lavergne and Molofsky 2007), or other attributes that improve colonization or competitiveness with native species (eg. Jain and Martins 1979; Prati and Bossdorf 2004; Kliber and Eckert 2005; Dlugosch and Parker 2008a, 2008b; Seifert et al. 2009, Xu et al. 2010).
Hybridization between species or genetically distinct populations of the same species can be an important factor leading to evolutionary change and successful invasions (Arnold 1997; Ellstrand and Schierenbeck 2000; Rieseberg et al. 2007; Schierenbeck and Ellstrand 2009). For instance, hybridization may produce novel genotypes that have a selective advantage in the introduced range. This could arise through producing traits that are intermediate between the two parents, recombining parental traits, or by producing transgressive (extreme) traits, some of which might be favorable in the new environment (Ellstrand and Schierenbeck 2000; Rieseberg et al. 2007). Hybridization also may be particularly advantageous in exotic plant populations because there will be an initial lack of local adaptation. The benefits of hybridization such as heterosis or the production of novel genotypes may then outweigh the cost of losing gene combinations for local adaptation in the former native range (Verhoeven et al. 2011). There are now a number of examples of inter- and intra-specific hybridization that have led to invasiveness (Schierenbeck and Ellstrand 2009; Gaskin et al. 2009; Travis et al. 2010; Mukherjee et al., pers. comm.).
Brazilian peppertree (Schinus terebinthifolius Raddi, Anacardiaceae) was introduced into Florida from South America in the 19th century as an ornamental plant (Morton 1978). Based on herbarium records and available literature, it began to escape cultivation in the 1950s, and is currently one of Florida’s most invasive weeds (Schmitz et al. 1991; Cuda et al. 2004, 2006). The initial discovery of a naturalized population of Brazilian peppertree in the Florida Keys 50–60 years after it was introduced confirms the long lag period often exhibited by woody weeds before they become invasive (Kowarik 1995). Lag periods for exponential growth and naturalization of horticultural plants like Brazilian peppertree can be correlated with marketing time (Pemberton and Liu 2009), and/or may be a function of propagule pressure and evolutionary change after introduction (Sakai et al. 2001).
Historical records and genetic evidence indicate that two genetic lineages of Brazilian peppertree were established in Florida, USA, one in Miami on the east coast and a second near Punta Gorda on the west coast (Nerhling 1944; Morton 1978; Workman 1979). Since arriving, the distributions of these two genotypes have greatly expanded, and they have extensively hybridized (Williams et al. 2005, 2007).
Brazilian peppertree was initially targeted for biological control in Hawaii (Krauss 1963; Yoshioka and Markin 1991), and later in Florida, USA (Bennett et al. 1990; Cuda et al. 2006). Recent surveys for natural enemies in Brazil resulted in the discovery of several insects, specifically thrips and psyllids, that are highly adapted to the two Brazilian peppertree genotypes and/or to their hybrids established in Florida (Manrique et al. 2008; Christ 2010). More importantly, natural enemies belonging to the thrips genus Pseudophilothrips and the psyllid genus Calophya turned out to be a complex of cryptic species (Cuda et al. 2009; Mound et al. 2010; Burckhardt et al. 2011).
In this review, the ecological significance of local adaptation of these natural enemies is discussed in the context of the different Brazilian peppertree populations and their hybrids.
Genetic structure of Brazilian peppertree
Both chloroplast sequence and nuclear microsatellite data indicate there were two separate introductions of Brazilian peppertree into Florida, one on the west coast (the A chloroplast haplotype) and one on the east coast (the B chloroplast haplotype) (Williams et al. 2005, 2007). GIS mapping indicated that individuals with the highest ancestry were found close to the introduction sites and became progressively more admixed with increasing distance away from the introduction sites (Williams et al. 2007). Although we do not know when the two lineages began hybridizing, most Brazilian peppertrees in Florida are now hybrids although there are still individuals in the Miami and Punta Gorda areas that retain a high proportion of ancestral DNA (ancestry coefficient, q > 0.90) from the original introductions (Williams et al. 2007).
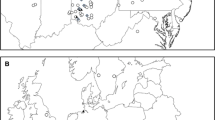
Brazilian peppertree has strong phylogeographic structure in its native range with most localities having only one or several closely related chloroplast haplotypes differing by one to two mutational differences (Williams et al. 2005). A parsimony network revealed that the introduced haplotypes A and B were different from other haplotypes in Brazil (Fig. 1). Furthermore, haplotypes A and B are from allopatric populations, indicating that the Florida introductions are from two distinct regions in South America. The origin of the Punta Gorda haplotype A is southeastern Brazil (Williams et al. 2005), whereas the origin of the Miami haplotype B is northeastern Brazil (Williams, unpublished).
CpDNA haplotype network of Schinus terebinthifolius illustrating relationships between the different haplotypes. Each connecting line indicates one nucleotide difference and unlabeled nodes are inferred intermediates. Figure redrawn from Williams et al. (2005)
Genetic structure of natural enemies
Pseudophilothrips spp.
An extensive investigation of the genetic structure of P. ichini and a recently described cryptic species Pseudophilothrips gandolfoi Mound, Wheeler and Williams (Mound et al. 2010) was conducted using the mitochondrial cytochrome oxidase I gene (COI). Seven haplotypes were found in P. ichini from Bahia to Santa Catarina, Brazil, but only a single haplotype was found for the recently described P. gandolfoi (Fig. 2), which appears to be confined to more inland populations of Brazilian peppertree in the state of Paraná (Mound et al. 2010). P. gandolfoi, previously referred to as Pseudophilothrips sp. near ichini (Manrique et al. 2008), is almost always associated with Brazilian peppertree populations characterized by haplotypes C and D and has very low survival on populations characterized by haplotype A from Brazil (Manrique et al. 2008).
Relationships of Pseudophilothrips species inferred using the neighbor-joining method and K2P pairwise distances of mitochondrial COI sequences. Bootstrap values are shown next to the branches. The scale bar indicates the number of base substitutions per site. Figure from Mound et al. (2010); reprinted with permission
Calophya spp.
To date, the genetic structure of only a small sample of C. terebinthifolii from Santa Catarina (n = 20) was investigated with the COI using methods similar to those reported in Mound et al. (2010). During a recent survey trip in March 2010, psyllids collected on Brazilian peppertree in northeastern Brazil (Salvador, Bahia) were identified as a new species, Calophya latiforceps Burckhardt, using both morphological and molecular characters (Burckhardt et al. 2011).
After sequencing this new species (n = 4 individuals) at the COI gene and calculating the Kimura 2-parameter (K2P) genetic distance both between and within the two psyllid species Kimura (1980), the results indicated that all individuals tested from southern Brazil have identical mitochondrial COI haplotypes and that the four psyllids sequenced from Salvador only differ at 0.2–0.7% of their sequence. However, C. latiforceps from Salvador, Bahia, was found to be genetically different from C. terebinthifolii collected in southern Brazil with a 13.4% sequence divergence. The morphological and genetic evidence confirmed the two psyllids are distinct species (Burckhardt et al. 2011).
Performance of natural enemies on Brazilian peppertree genotypes
Pseudophilothrips spp.
Performance (survival, development time, and adult longevity) of P. ichini and P. gandolfoi has been investigated in the laboratory (Manrique et al. 2008). P. ichini was originally collected on Brazilian peppertrees in the city of Ouro Preto, Minas Gerais, Brazil in November 2007. Peppertrees in this region are characterized by haplotype A. In contrast, P. gandolfoi was collected on Brazilian peppertrees in the vicinity of Curitiba, Paraná, Brazil, in January 2007. Peppertrees here carry either haplotype C or D. Ouro Preto is located 830 km northeast of Curitiba.
The two Pseudophilothrips spp. differed in their ability to accept the Florida populations as their host plants. For instance, P. gandolfoi exhibited low survival (0–4%) and short adult longevity (<ten days) when reared on the original Florida populations characterized by haplotypes A and B, or their hybrids between the original invasive populations, whereas higher survival (~50%) and longevity (~30 days) were observed for P. ichini on these same haplotypes (Manrique et al. 2008).
Calophya spp.
Performance (% rearing success) of C. terebinthifolii was investigated on five native haplotypes of Brazilian peppertree in a laboratory study conducted in Brazil (Christ 2010). Calophya terebinthifolii performed significantly better on plants with haplotype A, which occur in Florida, than the other populations characterized by haplotypes O, D, K, and M (G-test, G = 7.63; P < 0.01) (Christ 2010). There was over a 75% success rate when this psyllid was raised on its natal plants with haplotype A, a 20% success rate on plants with haplotype O, and 0% success on plants characterized by haplotypes D, K, and M (Fig. 3). Because haplotypes M and O are only one base-pair different from haplotype K (D.A. Williams, unpublished data), these data suggest that populations of C. terebinthifolii also are highly adapted to specific Brazilian peppertree haplotypes. The performance of the newly described C. latiforceps from Salvador has not yet been tested on the different Brazilian peppertree haplotypes.
Performance of Calophya terebinthifolii on five different haplotypes of Brazilian peppertree. Data from Christ (2010). Sample sizes for haplotypes were A (n = 12), O (n = 5), K (n = 2), D (n = 2), and M (n = 1)
Conclusions
Most of Florida’s Brazilian peppertrees are the result of intraspecific hybridization between two introductions (haplotypes A and B) from distinct source regions in Brazil (Williams et al. 2005, 2007; Mukherjee et al., pers. comm.). Common garden studies recently conducted in Florida suggest that these hybrid individuals have higher growth rates than the parental types, which may have facilitated the invasion (Geiger et al. 2011). Our research on the genetics and performance of two thrips and two psyllid natural enemies of Brazilian peppertree has shown that these insects exhibit what Harley and Forno (1992) referred to as ‘fine tuned’ adaptation to specific populations and genotypes of their host plants. On-going studies have not yet revealed a negative effect of hybridization per se on the performance of biological control agents. However, our studies indicate that it may be necessary to match biocontrol agents with the specific Brazilian peppertree geographic populations and/or their hybrids that occur in the introduced range.
Successful invasive species often are introduced multiple times from distinct source regions in the native range, so intraspecific hybridization may be quite common (Dlugosch and Parker 2008a, 2008b; Schierenbeck and Ellstrand 2009). Several studies also have shown that some pathogens and herbivores can be adapted to specific genotypes or populations of their host plants (Hasan 1972; Karban 1989; Karban and Strauss 1994; Gaskin and Schaal 2002; Goolsby et al. 2006; Ayres et al. 2009; Gaskin and Kazmer 2009). Our studies on the Brazilian peppertree invasion in Florida and fine scale adaptation of some of its natural enemies provide further evidence of this phenomenon. Future biological control programs could benefit from population genetic studies on both the invasive species and potential biological control agents, especially when the specific origin(s) of the weed are in doubt or there is evidence of differential attack by natural enemies.
References
Arnold ML (1997) Natural hybridization and evolution. Oxford University Press, Oxford
Ayres DR, Ryan FJ, Grotkopp E, Bailey J, Gaskin J (2009) Tumbleweed (Salsola, section Kali) species and speciation in California. Biol Invasions 11:1175–1187
Baker HG (1965) Characteristics and modes of origins of weeds. In: Baker HG, Stebbins GL (eds) The genetics of colonizing species. Academic Press, New York, pp 147–172
Bennett FD, Crestana L, Habeck DH, Berti-Filho E (1990) Brazilian peppertree—prospects for biological control. In: Delfosse ES (ed) Proceedings of the VII International Symposium on biological control of weeds, pp 6–11
Blossey B, Nötzold R (1995) Evolution of increased competitive ability in invasive nonindigenous plants–a hypothesis. J Ecol 83:887–889
Burckhardt D, Cuda JP, Manrique V, Diaz R, Overholt WA, Williams DA, Christ LR, Vitorino MD (2011) Calophya latiforceps, a new species of jumping plant lice (Hemiptera: Calophyidae) associated with Schinus terebinthifolius (Anacardiaceae) in Brazil. Florida Entomol 94:489–499
Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443
Christ LR (2010) Biology, population growth, and feeding preferences of Calophya terebinthifolii (Hempitera: Psyllidae), a candidate for biological control of Brazilian Peppertree, Schinus terebinthifolius (Anacardiaceae). MS Thesis, University of Florida, USA
Cox GW (2004) Alien species and evolution: the evolutionary ecology of exotic plants, animals, microbes and interacting native species. Island Press, Washington DC, USA
Cuda JP, Habeck DH, Hight SD, Medal JC, Pedrosa-Macedo JH (2004) Brazilian peppertree, Schinus terebinthifolius: Sumac family-Anacardiaceae. In: Coombs E, Clark J, Piper G, Cofrancesco A (eds) Biological control of invasive plants in the United States. Oregon State University Press, Corvallis, pp 439–441
Cuda JP, Ferritier AP, Manrique V, Medal JC (2006) Brazilian peppertree management plan for Florida: recommendations from the Brazilian Peppertree Task Force, Florida Exotic Pest Plant Council, 2nd edn. http://www.fleppc.org/Manage_Plans/2006BPmanagePlan5.pdf
Cuda JP, Medal JC, Gillmore JL, Habeck DH, Pedrosa-Macedo JH (2009) Fundamental host range of Pseudophilothrips ichini sensu lato (Thysanoptera: Phlaeothripidae), a candidate biological control agent of Schinus terebinthifolius (Sapindales: Anacardiaceae) in the USA. Environ Entomol 38:1642–1652
Dlugosch KM, Parker IM (2008a) Invading populations of an ornamental shrub show rapid life history evolution despite genetic bottlenecks. Ecol Lett 11:701–709
Dlugosch KM, Parker IM (2008b) Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17:431–449
Ellstrand NC, Schierenbeck KA (2000) Hybridization as a stimulus for the evolution of invasiveness in plants? Proc Nat Acad Sci USA 97:7043–7050
Elton CS (1958) The ecology of invasion by animals and plants. T Metheun and Co., London
Gaskin JF, Kazmer DJ (2009) Introgression between saltcedars (Tamarix chinensis and T. ramosissima) in the USA invasion. Biol Invasions 11:1121–1130
Gaskin JF, Schaal BA (2002) Hybrid Tamarix widespread in US invasion and undetected in native Asian range. Proc Nat Acad Sci USA 99:11256–11259
Gaskin JF, Wheeler GS, Purcell MF, Taylor GS (2009) Molecular evidence of hybridization in Florida’s sheoak (Casuarina spp.) invasion. Mol Ecol 18:3216–3226
Geiger JH, Pratt PD, Wheeler GS (2011) Hybrid vigor for the invasive exotic Brazilian peppertree (Schinus terebinthifolius Raddi., Anacardiaceae) in Florida. Int J Plant Sci 172:655–663
Goolsby JA, de Barro PJ, Makinson JR, Pemberton RW, Hartley DM, Frohlich DR (2006) Matching the origin of an invasive weed for selection of a herbivore haplotype for a biological control programme. Mol Ecol 15:287–297
Harley KLS, Forno IW (1992) Biological control of weeds: a handbook for practitioners and students. Inkata Press, Melbourne
Hasan S (1972) Specificity and host specialization of Puccinia chondrillina. Ann Appl Biol 72:257–263
Jain SK, Martins PS (1979) Ecological genetics of the colonizing ability of rose clover (Trifolium hirtum All). Am J Bot 66:361–366
Karban R (1989) Fine-scale adaptation of herbivorous thrips to individual host plants. Nature 340:60–61
Karban R, Strauss SY (1994) Colonization of new host plant individuals by locally adapted thrips. Ecography 17:82–87
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
Keller SR, Sowell DR, Neiman M, Wolfe LM, Taylor DR (2009) Adaptation and colonization history affect the evolution of clines in two introduced species. New Phytol 183:678–690
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kliber A, Eckert CG (2005) Interaction between founder effect and selection during biological invasion in an aquatic plant. Evolution 59:1900–1913
Kowarik I (1995) Time lags in biological invasion with regard to the success and failure of alien species. In: Pysek P, Rejmánek M, Wade M (eds) Plant invasions-general aspects and special problems. The Netherlands, SPB Academic Publishing, Amsterdam, pp 15–38
Krauss NL (1963) Biological control investigations on Christmas berry (Schinus terebinthifolius) and Emex (Emex spp.). Proc Hawaii Entomol Soc 18:281–287
Lavergne S, Molofsky J (2007) Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proc Nat Acad Sci USA 104:3883–3888
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17:386–391
MacArthur RH (1970) Species packing and competitive equilibrium for many species. Theor Popul Biol 1:1–11
Manrique V, Cuda JP, Overholt WA, Williams D, Wheeler G (2008) Effect of host-plant genotypes on the performance of two candidate biological control agents of Brazilian peppertree in Florida. Biol Control 47:167–171
Maron JL, Vila M, Bommarco R, Elmendorf S, Beardsley P (2004) Rapid evolution of an invasive plant. Ecol Monogr 74:261–280
Maron JL, Elmendorf SC, Vilà M (2007) Contrasting plant physiological adaptation to climate in the native and introduced range of Hypericum perforatum. Evolution 61:1912–1924
Morton JF (1978) Brazilian pepper–its impact on people, animals and the environment. Econ Bot 32:353–359
Mound LA, Wheeler GS, Williams DA (2010) Resolving cryptic species with morphology and DNA; thrips as a potential biocontrol agent of Brazilian peppertree, with a new species and overview of Pseudophilothrips (Thysanoptera). Zootaxa 2432:59–68
Nerhling H (1944) My garden in Florida. American Eagle, Estero
Parker IM, Rodriguez J, Loik ME (2003) An evolutionary approach to understanding the biology of invasions: local adaptation and general-purpose genotypes in the weed Verbascum thapsus. Cons Biol 17:59–72
Pemberton RW, Liu H (2009) Marketing time predicts naturalization of horticultural plants. Ecology 90:69–80
Pimentel D (2002) Biological invasions: economic and environmental costs of alien plant, animal and microbe species, CRC Press LLC, Boca Raton
Prati D, Bossdorf O (2004) Allelopathic inhibition of germination by Alliaria petiolata (Brassicaceae). Am J Bot 91:285–288
Prentis PJ, Wilson JRU, Dormontt EE, Richardson DM, Lowe AJ (2008) Adaptive evolution in invasive species. Trends Plant Sci 13:288–294
Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M (2006) Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol Lett 9:981–993
Rieseberg LH, Kim SC, Randell RA, Whitney KD, Gross BL, Lexer C, Clay K (2007) Hybridization and the colonization of novel habitats by annual sunflowers. Genetica 129:149–165
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Ann Rev Ecol Syst 32:305–332
Schierenbeck K, Ellstrand N (2009) Hybridization and the evolution of invasiveness in plants and other organisms. Biol Invasions 11:1093–1105
Schmitz DC, Simberloff D, Hofstetter RH, Haller W, Sutton D (1997) The ecological impact of nonindigenous species. In: Simberloff D, Schmitz DC, Brown TC (eds) Strangers in paradise: impact and management of nonindigenous species in Florida. Island Press, Washington DC, pp 9–61
Seifert EK, Bever JD, Maron JL (2009) Evidence for the evolution of reduced mycorrhizal dependence during plant invasion. Ecology 90:1055–1062
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biol Invasions 1:21–32
Suarez AV, Tsutsui ND (2008) The evolutionary consequences of biological invasions. Mol Ecol 17:351–360
Travis SE, Marburger JE, Windels S, Kubátová B (2010) Hybridization dynamics of invasive cattail (Typhaceae) stands in the Western Great Lakes Region of North America: a molecular analysis. J Ecol 98:7–16
Verhoeven KJF, Macel M, Wolfe LM, Biere A (2011) Population admixture, biological invasions and the balance between local adaptation and inbreeding depression. Proc Roy Soc B 278:2–8
Williams JR (1954) The biological control of weeds In: Rept Sixth Commonwealth Entomol Congress, London, UK, pp 95–98
Williams DA, Overholt WA, Cuda JP, Hughes CR (2005) Chloroplast and microsatellite DNA diversities reveal the introduction history of Brazilian peppertree (Schinus terebinthifolius) in Florida. Mol Ecol 14:3643–3656
Williams DA, Muchugu E, Overholt WA, Cuda JP (2007) Colonization patterns of the invasive Brazilian peppertree, Schinus terebinthifolius, in Florida. Heredity 98:284–293
Williamson M (1996) Biological invasions. Chapman & Hall, London
Workman R (1979) History of Schinus in Florida. In: Workman R (ed) Schinus—technical proceedings of techniques for control of Schinus in South Florida: a workshop for natural area managers. The Sanibel-Captiva Conservation Foundation Inc., Sanibel, pp 3–6
Xu CY, Julien MH, Fatemi M, Girod C, Van Klinken RD, Gross CL, Novak SJ (2010) Phenotypic divergence during the invasion of Phyla canescens in Australia and France: evidence for selection-driven evolution. Ecol Lett 13:32–44
Yoshioka ER, Markin GP (1991) Efforts of biological control of Christmas Berry Schinus terebinthifolius in Hawaii. In: Center T, Doren RF, Hofstetter RL, Myers RL, Whiteaker LD (eds) Proceedings, Symposium of Exotic Pest Plants, 2–4 November 1988, Miami, Florida, pp 377–385
Acknowledgments
We thank two anonymous reviewers for their comments on an earlier draft of this manuscript. These projects were supported by grants from the Florida Department of Environmental Protection, South Florida Water Management District, and Florida Exotic Pest Plant Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Ted Douglas
Rights and permissions
About this article
Cite this article
Cuda, J.P., Christ, L.R., Manrique, V. et al. Role of molecular genetics in identifying ‘fine tuned’ natural enemies of the invasive Brazilian peppertree, Schinus terebinthifolius: a review. BioControl 57, 227–233 (2012). https://doi.org/10.1007/s10526-011-9418-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-011-9418-y