Abstract
Fruit flies are pests of great economic importance due to their quarantine pest status and losses recorded in West Africa. An inventory of parasitoids associated with fruit flies in mangoes, guavas, cashew, pepper and major wild fruit crops was carried out in northern-central Benin in 2005, 2006, and 2008. Tephritid parasitoids reared from field-collected fruits belonged to three families: Braconidae (97.2%), Eulophidae (1.6%) and Pteromalidae (1.2%). Fopius caudatus (Szépligeti) accounted for 73.8% of all the parasitoids and therefore was the most abundant and widely distributed parasitoid. The parasitism rate was 7.7%, with the highest recorded in wild fruit crop habitat. Ceratitis cosyra (Walker) (77%) was the fly host most commonly reared from fruits that produced F. caudatus. The recently introduced pest Bactrocera invadens Drew Tsuruta and White was rarely parasitized and only by Pachycrepoideus vindemmiae (Rondani) (Hymenoptera: Pteromalidae) at this time. This is the first report of the inventory of one native parasitoid species from B. invadens in Africa, especially in West Africa.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Fruit flies (Diptera: Tephritidae) are among the most economically important pests of edible fruits worldwide (Wharton 1989; Billah et al. 2008a; Vayssières et al. 2008) and are also considered to be pests of quarantine importance. Their control has been the focus of many fruit fly research programs, especially for biological control through the use of parasitic Hymenoptera (Clausen 1978; Waterhouse 1993; Ovruski et al. 2000). Numerous studies have demonstrated the feasibility of the use of parasitoids for fruit fly suppression (Clausen et al. 1965; Harris et al. 2000; Sivinski et al. 2000; Vargas et al. 2007). Parasitoids from Africa, including several species of Psyttalia and Fopius, have attracted much interest for use as biological control agents (Silvestri 1913; Messing 1999; Wharton et al. 2000; Lopez et al. 2003; Sime et al. 2006; Billah et al. 2008b). One species, Psyttalia concolor (Szépligeti), has been widely released throughout the Mediterranean region through various augmentative biological control programs (Raspi 1995).
In tropical regions, mango (Mangifera indica L.) is a particularly important tropical fruit for Sub-Saharan African national and regional economies, as well as for exports (Vayssières et al. 2008). Mangoes are attacked by both native and introduced tephritid fruit flies which wreak great economic devastation in both East Africa (Ekesi et al. 2006) and West Africa (Vayssières et al. 2008). Before 2003, native tephritid pests such as Ceratitis cosyra (Walker) destroyed an average of 40% of the total mango crop produced yearly in Africa (1.9 Mt) (Lux et al. 2003). In Benin for instance, at the end of the crop year, in 2005 as in 2006, over 75% of production was lost due to fruit flies (Vayssières et al. 2009). The arrival and dispersion of Bactrocera invadens Drew, Tsuruta and White throughout West Africa in 2004 has considerably worsened the damage to marketable fruit crops, especially mangoes (Vayssières et al. 2005).
The African continent has been the focus of several biological control programs, with a particular focus on collections and surveys of parasitoids for export to other regions to control pests such as Mediterranean fruit fly, Ceratitis capitata (Wiedemann) and the olive fly, Bactrocera oleae (Rossi) (Neuenschwander 1982; Steck et al. 1986; Wharton et al. 2000; Mkize et al. 2008; Wang et al. 2009; Rugman-Jones et al. 2009). Although some of the previous surveys have included Benin and adjacent countries (Silvestri 1914; Steck et al. 1986), mangoes were not included among the fruits sampled, and throughout Sub-Saharan Africa, there has been little effort to date to survey natural enemies attacking tephritid pests of mangos. The objectives of this preliminary study were (i) to make an inventory of fruit fly parasitoids in areas of large mango productions in Benin, and (ii) to determine the overall parasitism rate and the pest control potential of these Hymenopteran parasitoids to provide baseline data for future biological control efforts.
Materials and methods
Sampling sites
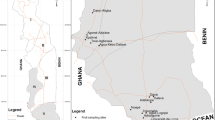
The most numerous and largest orchards (75% of Beninese mango orchards), in terms of area and diversity with 29 mango cultivars (Vayssières et al. 2008), are in Borgou. There we can find the greatest plant diversity as this zone is at the boundary of the Northern Guinean savannah and the Southern Sudanian savannah of Benin (Vayssières et al. 2008). The climate type of this zone is Sudanian, characterized by unimodal rainfall (1000–1100 mm yearly) with an annual mean temperature ranging from 26°C to 27°C. The rainy season usually starts at the end of April and lasts for six months until the end of October. The trials were conducted in this area at four different locations, Tchaourou, Parakou, N’Dali and Bembéréké, of this Borgou Department.
Fruit crop species
Fruits were sampled from different mango (M. indica) cultivars, namely, cv Ruby, Alphonse de Goa, Brooks, Eldon, Kent, Keitt, Dabshar Drahnet, Smith, Zill, Gouverneur, Améliorée du Cameroun, Atacora and ungrafted local. These cultivars were selected for the study because they were present in almost all selected orchards. Other fruit crop species were also included in the fruit sampling and concerned wild fruit crop species, namely, Sarcocephalus latifolius (Smith) Bruce (Rubiaceae), Vitellaria paradoxa Gaertn. (Sapotaceae), Annona senegalensis Pers. (Annonaceae), and cultivated edible species, namely, Anacardium occidentale L. (Anacardiaceae), Psidium guajava L. (Myrtaceae), Citrus sinensis L. (Rutaceae) and Capsicum annuum L. (Solanaceae). These fruit species were chosen because they grow in very close proximity to these mango orchards. We have very similar habitats around these mango orchards.
Fruit sampling and observation in the laboratory
To determine the fly species and associated parasitoids in the selected fruit habitats, all mango cultivars as well as other cultivated and wild fruit crops encountered were sampled in a two-year preliminary survey during the fruit season in 2005 and 2006 and also in a more exhaustive survey in 2008. Samples were not taken throughout the year but only during seasons when the mentioned host fruits were available to sample (though other fruit hosts might have been available to the flies at other times of the year). In 2005, no survey samples were taken in January, February, September, October, November, December. In 2006, no survey samples were taken in January, August, September, October, November, December. In 2008, no survey samples were taken in January, February, March, August, September, October, November, December. In the preliminary survey, fruits at prematurity and maturity stages were randomly sampled each week according to availability and reared in the laboratory to record the fly species and associated parasitoids. In the more exhaustive quantitative survey, data on fruit infestation rate, parasitism rate and parasitoid abundance were obtained.
Fruit samples were brought to the laboratory, individually weighed, counted and classified by cultivar, date and sample site. After being allocated a sequence number, they were placed for observation onto mesh supports mounted on basins filled with wet sand, in which larvae emerging from the fruits could drop and metamorphose into pupae (Vayssières et al. 2007). For each cultivar, the fruit samples were individualized according to the site and their sampling date for easy referencing of the sample origin. At five-day intervals, the sand covering the bottom of the containers was sieved to collect the pupae. The pupae, collected with flexible tweezers, were then put with a sequence number into small hatchery boxes lined with moist blotting paper. Newly emerged adults were removed every three days. Fruit fly identifications were made by J.F. Vayssières but confirmed by M. De Meyer in some cases. Parasitoid identifications were made by R. Wharton in the laboratories of Texas A&M University, USA. Voucher specimens are deposited in the Insect Collections in Texas A&M University with references numbers RVA 1643-1671, RVA 1772-1798, RVA 2062-2147.
Percent parasitism was calculated as a/(a + b) × 100, where a = number of recovered parasitoids and b = number of emerged adult flies in each sample (Steck et al. 1986). Infestation rate was calculated as the number of pupae per kg fruit. Log10 (x + 1) transformation was used on percentage data to stabilize the variance and normalize the data. Analysis of variance was performed using the general linear model procedure and mean separations were done using the Student–Newman–Keuls test (SAS 2003).
Host/parasitoid associations are based on assumptions that parasitoids reared from a fruit sample were attacking only hosts that were also reared as adults from that sample, with attack rate on different hosts based on percent of hosts in the sample. We can consider that this is only an approximation, and that many factors, such as the ability of parasitoids to select preferred hosts and encapsulation by unsuitable hosts, increase the level of uncertainty in this regard.
Results
The numbers of sampled fruits per crop species per cultivar during the full survey in 2008 are shown in Table 1. There were 810 fruits collected every week, with a total of 6970 fruits collected and held in the laboratory for rearing and emergences of insects. Six species of parasitoids belonging to three families of Hymenoptera were reared from survey samples (Table 2): Braconidae [Fopius caudatus (Szépligeti), Psyttalia cosyrae (Wilkinson), P. perproxima (Silvestri), and Diachasmimorpha fullawayi (Silvestri)], Eulophidae (Tetrastichus giffardianus Silvestri) and Pteromalidae (Pachycrepoideus vindemmiae (Rondani). The majority of the parasitoids were braconids (97.2%). The most abundant parasitoid was F. caudatus (73.8%) followed by Psyttalia spp (21.8%) (with P. perproxima 19.5% and P. cosyrae 2.3%). These proportions are based on the total samples studied (data not shown). Fopius and Psyttalia were recorded in all fruit crop habitats except those of P. guajava and C. annuum. The occurrence rate for all other parasitoid species combined was less than 5%.
Factors such as the location (Fig. 1), habitat (fruit crop species) (Table 2, Fig. 2), fruit fly species (Table 3) and fruit seasons (Table 4) determine the occurrence and parasitism rate of the parasitoid species. Thus, parasitism rate was the highest (F = 4.745, df = 3, 96; P = 0.024) in Bembéréké (8.94%) while the lowest rate was recorded in Parakou (3.21%) (Fig. 1). Infestation and parasitism rates were also cultivar-dependent (Table 2). Comparing all fruit crops included in the study, cultivated fruit crops showed the significantly (F = 3.760; df = 16, 248; P = 0.041) lower parasitism rates as compared to wild fruit crop species such as A. senegalensis and S. latifolius. For instance, the parasitism rate recorded in M. indica habitat was 5.9% (irrespective to the cultivars) which was significantly (F = 3.760; df = 16, 248; P = 0.041) lower than that of wild fruit crop species (Fig. 2). However, the parasitism rate in general was less than 30% (Fig. 2). No correlation was detected between fruit weight and infestation rate (r 2 = 0.157, F = 1.045, df = 1, 264; P = 0.958) or fruit weight and parasitism (r 2 = 0.101, F = 0.495, df = 1, 264; P = 0.186). However, higher (F = 19.325; df = 16, 248; P = 0.036) parasitism rates are recorded from wild fruits such as A. senegalensis (20.40 ± 2.37) and S. latifolius (28.90 ± 1.84), which also had the highest (F = 19.325; df = 16, 248; P = 0.036) infestation rates (Table 2).
For the fruit fly species/parasitoid samples identified, the fruit fly host most frequently reared from fruit samples that produced parasitoids was C. cosyra (77% of all flies reared from fruit samples producing tephritid parasitoids were C. cosyrae). The least parasitized were B. invadens (5.1%), C. quinaria (Bezzi) (5.1%) and C. silvestrii (0.8%) (Table 3). C. cosyra, the most damaging fruit fly pest in northern-central Benin, is mostly attacked by F. caudatus as its principal parasitoid. It is also secondarily parasitized by P. cosyrae and to a lesser extent by D. fullawayi (Table 3). F. caudatus and P. cosyrae also appeared to be the principal parasitoids of C. quinaria. In the samples processed to date, C. silvestrii Bezzi was only parasitized by P. perproxima and B. invadens was only parasitized by P. vindemmiae. Our preliminary data suggest that parasitism rates are the highest in June (Table 4).
Discussion
To our knowledge, this is the first inventory of parasitoid species from cultivated mangoes in Benin and provides critical baseline data for future conservation or introduction of parasitoids in biological control programs. The six parasitoid species have been reported from West Africa previously (Vayssières et al. 2002). There are several species of Psyttalia in the Sub-Saharan region that are difficult to differentiate (Rugman-Jones et al. 2009). The species reared in this program include both long-ovipositor forms (P. cosyrae) and some with shorter ovipositors that we are calling P. perproxima. P. perproxima was originally described from Benin from fruits infested by tephritids (Silvestri 1913), and our material fits the original description of this species. However, because the species of Psyttalia are difficult to discriminate, it is possible that more than two species of Psyttalia are included in our samples, including either P. concolor or the nearly identical P. humilis (Silvestri) (Rugman-Jones et al. 2009). F. caudatus was also recorded by Silvestri (1913) from Benin and F. silvestrii was originally described by Wharton (1987) from West Africa (Togo) and Central Africa (Cameroon).
The majority of the recorded parasitoids from all the samples in the current study were braconids and F. caudatus was the most abundant and widely distributed, followed by Psyttalia spp. F. caudatus was recorded in different locations and from different fruit crops. Despite the numerous fruit fly species, such as C. cosyra, C. silvestrii, C. quinaria, C. capitata and B. invadens, recovered from fruits in the current experiment, F. caudatus was recovered most frequently from C. cosyra-infested fruits and secondarily from C. quinaria-infested fruits. These species might therefore be the preferred hosts for F. caudatus in our samples. As reported earlier, fruit fly parasitoids, in general, may develop on several tephritids but they often have a specific, preferred host (Wharton 1989). This was the case in Hawaii where Fopius arisanus (Sonan), a related species, caused substantial reduction of the host populations when released against the introduced pest Bactrocera dorsalis (Hendel), but became the major parasitoid of a previously introduced pest, C. capitata (Vargas et al. 2007). In the current study and for the fruit fly/parasitoid samples identified so far, the native parasitoid F. caudatus was not recovered from fruits infested by certain fruit flies such as C. capitata, C. silvestrii and B. invadens. Further controlled laboratory setting experiment will confirm whether these fruit fly species do not permit the development of F. caudatus.
As for any other insect, parasitoid survival and hence parasitism rate can be influenced by abiotic factors such as temperature (Rousse et al. 2005) and relative humidity. Therefore, samples from different agro-ecological zones may yield different recoveries of parasitoids. In our current work, the studied locations were in a similar agro-ecological zone and as such the climatic conditions might not be the driving factor explaining the difference found among samples in terms of recovered parasitoids and parasitism rate. Rather, the phenological stage of the fruit crops and the probable proximity of wild fruit species in each location may be an important factor influencing the occurrence of the parasitoids. The most infested phenological stages are mainly the pre-mature and mature fruits hosting eggs and larvae of Tephritidae. Wild fruit data of S. latifolius and A. senegalensis are indeed a kind of “parasitoid-pond” with higher parasitism rates of 28.9% and 20.4%, respectively (Table 2). During the current survey, samples were not taken throughout the year but only during seasons when the mentioned host fruits were available to sample (other fruit hosts might have been available to the flies at other times of the year). Most of the parasitoids were recovered in May–June or July, like their host flies, and this period coincided with the rainy season and the second half of the mango season.
The overall mean parasitism rate considering all samples was 6.36 ± 1.96% with the lowest rate 0.02 ± 0.00% recorded in mango cv Zill. Previous hypotheses have attributed low rates of parasitism in part to physical difficulties in locating immature stages of fruit flies within large fruits (Sivinski et al. 2000; Wang et al. 2009). Our results showed the lowest parasitism rate recorded from M. indica cultivars but the highest from the wild fruit crop species habitats such as A. senegalensis and S. latifolius where larvae are closer to the fruit surface.
In Table 2 we show that small size of fruits can be linked with higher parasitism rate than large size as mango fruits (with many cv). There was a negative correlation (y = −0.5614x + 1.8683; r 2 = 0.781; F = 7.135; df = 1, 32; P = 0.034) between the fruit size and the parasitism rate. These observations could support Sivinski’s and Wang’s work on ovipositor length vs fruit size. If it is true for A. senegalensis it is not evident for S. latifolius which hosts a large amount of F. caudatus. As they oviposit in fly eggs which are placed not so far from the surface of the fruit, then, fruit size is not really a problem for Fopius species. Anyway, we can generally say that we have a greater number and diversity larval parasitoid obtained from small wild fruits (A. senegalensis, V. paradoxa) than large cultivated grafted mangoes (Brooks, Dabschar, Smith, Zill).
On the other hand, cultivated crop habitats such as mango orchards may constitute unfavorable conditions due to human interventions (cultural practices, insecticide application) that negatively impact parasitoids. This is consistent with the report from Hernández-Ortiz et al. (2006). These authors attributed low level of parasitism in his study to probable orchard management practices, in which periodic pesticide use could have a negative impact on parasitoid populations. Studies carried out in Brazil reported similar species diversity and levels of parasitism (Uchôa-Fernandes et al. 2003). In either case, our data are consistent with earlier findings concerning parasitoid abundance in wild vs cultivated settings (Aluja and Liedo 1986; Vargas et al. 1993; Sivinski et al. 2000; Hernández-Ortiz et al. 2006) and indicate a host habitat preference for the wild fruit crop habitat for the dominant parasitoid species reported here. The presence of these wild hosts is of great importance for IPM in general, and especially for biological control (Vayssières et al. 2002). Higher infestation rates in wild fruits can serve as a source for infestations in cultivated fruits at least seasonally. However, these wild plants are also a potential reservoir for the development of the parasitoids throughout the year.
In a given fruit crop production area, there is always a diverse assemblage of native fruit fly parasitoids, and our samples indicate that northern-central Benin is no exception. Yet, the efficacy of this parasitoid assemblage in controlling tephritid pests is low as indicated by the high infestation rate with low parasitism rate. Our most abundant species, F. caudatus, exhibited generally low levels of parasitism overall, and was not recovered from the introduced pest B. invadens. This very low rate of parasitism by natives may justify the introduction of a non-native such as F. arisanus that is known to be very effective against many fruit flies (Haramoto and Bess 1970; Rousse et al. 2005, 2006) including members of the B. dorsalis complex. For example, F. arisanus has been introduced for a classical biological control program by CIRAD (International Centre of Agricultural Research for Development) into Reunion Island against B. zonata. Most recently, F. arisanus proved very successful when introduced to Tahiti (Vargas et al. 2007) against B. dorsalis. F. arisanus has been reported to parasitize 21 tephritid species and to develop, with variable success, on 18 of them (Rousse et al. 2005). Therefore, F. arisanus could similarly be effective against the damaging fruit flies in West Africa especially B. invadens.
References
Aluja M, Liedo P (1986) Future perspectives on integrated management of fruit flies in Mexico. In: Mangel M (ed) Pest control: operations and systems analysis in fruit fly management. Proceedings of a NATO advanced research workshop. Springer Verlag, New York, pp 12–48
Billah MK, Kimani-Njogu SW, Wharton RA, Overholt WA, Wilson DD, Cobblah MA (2008a) Cross mating studies among five fruit fly parasitoid populations: potential biological control implications for tephritid pests. Biol Control 53:709–724
Billah MK, Kimani-Njogu SW, Wharton RA, Woolley JB, Masiga D (2008b) Comparison of five allopatric fruit fly parasitoid populations (Psyttalia sp.) (Hymenoptera: Braconidae) from coffee fields using morphometric and molecular methods. Bull Ent Res 98:63–75
Clausen CP (1978) Introduced parasites and predators of arthropod pests and weeds: a world review. Agriculture Handbook, no. 480. United States Department of Agriculture, Washington, USA
Clausen CP, Clancy DW, Chock QC (1965) Biological control of the oriental fruit fly (Dacus dorsalis Hendel) and other fruit flies in Hawaii. Tech Bull no, 1322. United States Department of Agriculture, USA
Ekesi S, Nderitu PW, Rwomushana I (2006) Field infestation, life history and demographic parameters of Bactrocera invadens Drew, Tsuruta and White, a new invasive fruit fly species in Africa. Bull Entomol Res 96:379–386
Haramoto FH, Bess HA (1970) Recent studies on the abundance of the Oriental and Mediterranean fruit flies and the status of their parasites. Proc Hawai Entomol Soc 20:551–566
Harris EJ, Bautista RC, Spencer JP (2000) Utilization of the egg-larval parasitoid, Fopius (Biosteres) arisanus, for augmentative biological control of fruit flies. In: Tan KH (ed) Area-wide control of fruit flies and other insect pests. Penerbit Universiti Sains Malaysia, Pulau Pinang, Malaysia, pp 725–732
Hernández-Ortiz V, Delfin-Gonzalez H, Escalante-Tio A, Manrique-Saide P (2006) Hymenopteran parasitoids of Anastrepha fruit flies (Diptera: Tephritidae) reared from different hosts in Yucatan, Mexico. Fla Entomol 89:508–515
Lopez M, Sivinski J, Rendon P, Holler T, Bloem K, Copeland R, Trostle M, Aluja M (2003) Colonization of Fopius ceratitivorus, a newly discovered African egg-pupal parasitoid (Hymenoptera: Braconidae) of Ceratitis capitata (Diptera: Tephritidae). Fla Entomol 86:53–60
Lux SA, Ekesi S, Dimbi S, Mohamed S, Billah M (2003) Mango-infesting fruit flies in Africa: perspectives and limitations of biological approaches to their management. In: Neuenschwander P, Borgemeister C, Langewald J (eds) Biological control in IPM systems in Africa. CAB Int, Wallingford, pp 277–298
Messing RH (1999) The impact of nontarget control on the practice of biological control. In: Follett PA, Duan JJ, Norwell MA (eds) Nontarget impact of biological control. Kluwer Academic, Dordrecht, pp 45–55
Mkize N, Hoelmer KA, Villet MH (2008) A survey of fruit-feeding insects and their parasitoids occurring on wild olives, Olea europea spp. cuspidata, in the Eastern Cape of South Africa. Biocontrol Sci Techn 18:991–1004
Neuenschwander P (1982) Searching parasitoids of Dacus oleae in South Africa. Z für Angew Entomol 94:509–522
Ovruski S, Aluja M, Sivinski J, Wharton RA (2000) Hymenoptera parasitoids on fruit-infesting Tephritidae (Diptera) in Latin America and the southern United States: diversity, distribution, taxonomic status and their use in fruit fly biocontrol. Int Pest Manag Rev 5:81–107
Raspi A (1995) Lotta biologica in olivicoltura. Atti del convegno su techniche, norme equalità in Olivicoltura. Potenza, Italia, 15–17 December 1993
Rousse P, Harris EJ, Quilici S (2005) Fopius arisanus, an egg pupal parasitoid of Tephritidae Overview. Biocontrol News Inf 26:59–69
Rousse P, Gourdon F, Quilici F (2006) Host specificity of the egg pupal parasitoid Fopius arisanus (Hymenoptera: Braconidae) in La Reunion. Biol Control 37:284–290
Rugman-Jones PF, Wharton R, van Noort T, Stouthamer R (2009) Molecular differentiation of the Psyttalia concolor (Szépligeti) species complex (Hymenoptera: Braconidae) associated with olive fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae), in Africa. Biol Control 49:17–26
SAS (2003) The SAS System for Windows, version 9.1 computer program. SAS, Cary (NC)
Silvestri F (1913) Viaggio in Africa per cercare parassiti di mosche dei frutti. Bollettino del Laboratorio di Zoologia Generale e Agraria, Portici 8:1–164
Silvestri F (1914) Report of an expedition to Africa in search of the natural enemies of fruit flies (Trypaneidae), with descriptions, observations and biological notes. Bull Ent Agric Hawaii 3:1–176
Sime KR, Daane KM, Messing RH, Johnson MW (2006) Comparison of two laboratory cultures of Psyttalia concolor (Hymenoptera: Braconidae), as a parasitoid of the olive fruit fly. Biol Control 39:248–255
Sivinski J, Piñero J, Aluja M (2000) The distributions of parasitoids (Hymenoptera) of Anastrepha fruit flies (Diptera: Tephritidae) along an altitudinal gradient in Veracruz, Mexico. Biol Control 18:258–269
Steck GJ, Gilstrap FE, Wharton RA, Hart WG (1986) Braconid parasitoids of Tephritidae (Diptera) infesting coffee and other fruits in West-Central Africa. Entomophaga 31:59–67
Uchôa-Fernandes MA, Molina Da SRM, De oliveira I, Zucchi RA, Canal NA, Díaz NB (2003) Larval endoparasitoids (Hymenoptera) of frugivorous flies (Diptera, Tephritoidea) reared from fruits of the cerrado of the state of Mato Grosso do Sul, Brazil. Rev Brasileira Entomol 47:181–186
Vargas RI, Stark JD, Uchida GK, Purcell M (1993) Opiine parasitoids (Hymenoptera: Braconidae) of oriental fruit fly (Diptera: Tephritidae) on Kauai Island, Hawaii: islandwide relative abundance and parasitism rates in wild and orchard guava habitats. Environ Entomol 22:246–253
Vargas RI, Leblanc L, Putoa R, Eitam A (2007) Impact of introduction of Bactrocera dorsalis (diptera: tephritidae) and classical biological control releases of Fopius arisanus (hymenoptera: braconidae) on economically important fruit flies in French polynesia. J Econ Entomol 100:670–679
Vayssières J-F, Wharton R, Delvare G, Sanogo F (2002) Diversity and pest control potential of hymenopteran parasitoids of Ceratitis spp. on mangos in Mali. In: Proceedings of 6th International fuit fly symposium 6–10 May 2002, Stellenbosch, South Africa, pp 461–464
Vayssières J-F, Georgen G, Lokossou O, Dossa P, Akponon C (2005) A new Bactrocera species in Benin among mango fruit fly (Diptera Tephritidae) species. Fruits 60:371–377
Vayssières J-F, Sanogo F, Noussourou M (2007) Inventory of the fruit fly species (Diptera: Tephritidae) linked to the mango tree in Mali, and tests of integrated control. Fruits 62:329–341
Vayssières J-F, Korie S, Coulibaly O, Temple L, Boueyi SP (2008) The mango tree in northern central Benin: cultivar inventory, yield assessment, infested stages and loss due to fruit flies (Diptera Tephritidae). Fruits 63:1–8
Vayssières J-F, Korie S, Ayegnon D (2009) Correlation of fruit fly (Diptera Tephritidae) infestation of major mango cultivars in Borgou (Benin) with abiotic and biotic factors and assessment of damage. Crop Protect 28:477–488
Wang XG, Nadel H, Johnson MW, Daane KM, Hoelmer K, Walton VM, Pickett CH, Sime KR (2009) Crop domestication relaxes both top-down and bottom-up effects on a specialist herbivore. Basic Appl Ecol 10:216–227
Waterhouse DF (1993) Biological control: pacific prospects, supplement 2. ACIAR, Canberra
Wharton RA (1987) Changes in nomenclature and classification of some opiine Braconidae (Hymenoptera). Proc Entomol Soc Wash 89:61–73
Wharton RA (1989) Classical biological control of fruit-infesting Tephritidae. In: Robinson AS, Hooper G (eds) Fruit flies their biology, natural enemies and control, vol 3B. Elsevier, Amsterdam, pp 303–313
Wharton RA, Trostle MK, Messing RH, Copeland RS, Kimani-Njogu SW, Lux S, Overholt WA, Mohamed S, Sivinski J (2000) Parasitoids of medfly, Ceratitis capitata, and related tephritids in Kenyan coffee: a predominantly koinobiont assemblage. Bull Entomol Res 90:517–526
Acknowledgments
We are grateful to IITA, CIRAD Montpellier and CIRAD-Réunion for support. Thanks are also due to growers from Borgou (especially M. Walis Zoumarou) for collaboration. We also thank our MM. Issa Ouagoussounon, Soumanou Modjibou and Adamou Abdoulaye for the monitoring of infested fruits in Parakou. We also thank most sincerely two unnamed reviewers who read this document and made a number of relevant remarks.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Dirk Babendreier
This work is part of a regional program on fruit fly control strategies in West Africa, coordinated by Dr Jean-François Vayssières, IITA-Benin. We think it is important to first inventory the native parasitoid species before undertaking the fruit fly biocontrol and especially before carrying out any introduction of exotic species of parasitoids.
Rights and permissions
About this article
Cite this article
Vayssières, JF., Wharton, R., Adandonon, A. et al. Preliminary inventory of parasitoids associated with fruit flies in mangoes, guavas, cashew pepper and wild fruit crops in Benin. BioControl 56, 35–43 (2011). https://doi.org/10.1007/s10526-010-9313-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-010-9313-y