Abstract
The predatory bug Orius albidipennis (Reuter) (Hemiptera: Anthocoridae) has tremendous potential as a biological control agent, especially in its native range around the Mediterranean Basin and East Africa. The need to exploit native biological control agents is growing in importance as concerns over the introduction of non-native species continue to increase. However, little is known of the effects of different prey on development and reproduction of O. albidipennis compared with other species of Orius. Therefore, we compared the development, survival, reproductive biology, and prey consumption of O. albidipennis when fed eggs of Ephestia kuehniella Zeller, Tetranychus urticae Koch, and Trialeurodes vaporariorum (Westwood), and larvae of Gynaikothrips ficorum (Marchal), under laboratory of 26 ± 1°C, 60 ± 10% RH and 16L:8D photoperiod. Individuals were reared from the neonate stage until death on one of the four prey types. The type of prey had profound effects on all measured performance traits. The highest survival rate was recorded for nymphs that were fed on E. kuehniella eggs, while the lowest survival rate was observed for those fed on T. vaporariorum eggs. The shortest nymphal period was recorded for nymphs fed on E. kuehniella eggs, while the longest was measured for those fed on T. urticae eggs. During the nymphal period, O. albidipennis consumed significantly more eggs of T. urticae than other prey types, whereas the lowest number of consumed prey were eggs of E. kuehniella. Adult females and males consumed significantly more T. urticae eggs than other types of prey. However, Orius albidipennis females showed the highest fecundity when fed on E. kuehniella eggs, and the lowest when fed on T. vaporariorum eggs. Adult females and males that fed on G. ficorum larvae had significantly longer life spans compared with those fed other prey. Because of their relatively rapid development and high fecundity, O. albidipennis fed E. kuehniella eggs had a significantly higher net reproductive rate (Ro) and intrinsic rate of increase (r m ) than O. albidipennis fed other prey types. Overall, eggs of E. kuehniella were the most suitable diet for nymphs and adults of O. albidipennis. Although less suitable, O. albidipennis could survive and reproduce on the other prey types, which is a favourable attribute in biological control agents. These results on the effect of different prey types on development and reproduction of O. albidipennis will also contribute to the development of mass rearing programs for biological control agents in developing countries, such as Egypt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Generalist predatory insects are capable of attacking a diverse spectrum of prey species (Eubanks and Denno 2000) because they have the necessary phenotypical plasticity to adjust their biology to different food sources (Mendes et al. 2002). However different types of prey can substantially alter the development and reproduction of predators, which in turn affect population dynamics. Species in the genus Orius (Hemiptera: Anthocoridae) are generalist predators that attack eggs and immature stages of various arthropods, or small soft-bodied adult arthropods, including numerous important agricultural pest species (Bush et al. 1993; Riudavets 1995; Lee et al. 1996; Reitz et al. 2006; Butler and O’Neil 2007). Although they are polyphagous, Orius spp. show a preference for attacking larval and adult thrips (Thysanoptera) over other available prey (Kakimoto et al. 2006; Arnò et al. 2008; Xu and Enkegaard 2009). Consequently, they are considered promising and effective as biological control agents and have been used successfully in biological control programs in greenhouse and open-field cropping systems against various thysanopteran pests. In particular, Orius insidiosus (Say) has been released into sweet pepper and cucumber greenhouses in Europe to successfully control Thrips tabaci (Lindeman) and the invasive Frankliniella occidentalis (Pergande) (van den Meiracker and Ramakers 1991; Dissevelt et al. 1995; Sabelis and van Rijn 1997; Perdikis et al. 2008). In its area of origin, conservation of O. insidiosus is a key component of integrated pest management (IPM) programs for thrips in field grown crops (Funderburk 2009). Orius laevigatus (Fieber) is another species that has been widely employed in successful biological control programs in Europe (Chambers et al. 1993; Sanchez and Lacasa 2002; Coll et al. 2007). For these reasons, a number of Orius spp., in particular O. laevigatus and O. insidiosus, are mass produced for augmentative release by various commercial insectaries in Europe and North America (Cranshaw et al. 1996; van Lenteren et al. 1997; Lattin 1999), where they are routinely reared on eggs of the Mediterranean flour moth Ephestia kuehniella Zeller (Arijs and De Clercq 2004; Bonte and De Clercq 2008). However, mass production of Orius spp. has not been realized in most developing countries (van Lenteren et al. 1997).
While certain species of Orius are mass produced for augmentative biological control, growing concerns over the introduction of non-native species limits where any particular species may be deployed (van Lenteren et al. 2003; Louda et al. 2003). As a result there is increased interest in other species of Orius that could be deployed as biological control agents in their native ranges. This interest is reinforced by the recognition that biological control agents also must be well acclimatized to environments where they would be deployed (Cocuzza et al. 1997a).
One species of Orius of particular interest as a biological control agent is O. albidipennis (Reuter), which is frequently found in large numbers in various agricultural habitats throughout the Mediterranean basin, the Atlantic zone of Western Europe, and East Africa (Salim et al. 1987; Chyzik et al. 1995b; Cocuzza et al. 1997b; Hernandez and Stonedahl 1999; Fritsche and Tamò 2000). Chyzik and Ucko (2002) reported that O. albidipennis could control thrips in pepper fields in Israel. In Egypt, O. albidipennis is very common throughout much of the country, south to Wadi Halfa, in the desert, and in cultivated areas, especially in corn and cotton fields. It is usually found in flowers of plants infested with thrips, lepidopteran eggs or other small arthropods (Tawfik and Ata 1973; Zaki 1989).
Orius albidipennis is well adapted to high temperatures (Cocuzza et al. 1997a) and does not have a photoperiod induced reproductive diapause (Chyzik et al. 1995a) as do other species of Orius (van den Meiracker 1994). Rather, its abundance and activity is only limited by low temperatures (Chyzik et al. 1995a). On the basis of our observations in Egypt, O. albidipennis does not occur in the field before March, but its activity increases from April until the end of November. Because of its heat tolerance and lack of photoperiod induced diapause, it could be an ideal candidate for mass rearing and augmentative releases in subtropical and tropical areas. However, its use as a biological control agent has been hindered by a relative lack of information on its interactions with different prey and in determining the suitability of different prey diets for O. albidipennis is an obstacle in its mass production. Therefore, the aim of the current study was to determine the effect of a range of different prey types (i.e. eggs of E. kuehniella, Tetranychus urticae Koch and Trialeurodes vaporariorum [Westwood], and 2nd instars of Gynaikothrips ficorum [Marchal]) on the development, reproduction, longevity, and prey consumption of this predator. These types of data can provide important information for understanding predator population dynamics and estimating population level effects of predators on prey species.
Materials and methods
Rearing of O. albidipennis
A colony of O. albidipennis was established from nymphs and adults collected on sunflower plants (Helianthus annuus L.) at the Experimental Farm, Faculty of Agriculture, Suez Canal University at Ismailia Governorate, Egypt. Adults and nymphs were maintained in one litre plastic jars (10 cm diameter × 20 cm height), which were covered with muslin that was held in place by rubber bands. Each jar was provided with sufficient quantities of loose E. kuehniella eggs as a food supply for the enclosed predators (Cocuzza et al. 1997b) and a piece of cotton that had been soaked in a 10% honey solution. A part of bean pod (Phaseolus vulgaris L.) was provided in each jar as an oviposition substrate (Isenhour and Yeargan 1981). Bean pods with newly deposited eggs were removed and replaced daily, and kept in the previously described plastic jars. Jars were examined daily for emergence of O. albidipennis nymphs. Soon after hatching, nymphs were carefully transferred to new plastic jars provisioned with E. kuehniella eggs and small styrofoam balls to offer hiding places and reduce cannibalism (Sobhy et al. 2005). Field collected adults and nymphs were added on a regular basis to refresh the colony and to increase its genetic variation. Upon eclosion, adults were sexed and placed in new plastic jars, provisioned with the same type of prey and oviposition substrates. Colonies were maintained at 26 ± 1°C, 60 ± 10% RH and 16L:8D photoperiod (Sobhy et al. 2006).
Rearing of prey species
Ephestia kuehniella: Eggs of E. kuehniella were taken from the mass rearing line in the Public Service Centre of Biological Control, Faculty of Agriculture, Suez Canal University, Ismailia, Egypt. Ephestia kuehniella larvae were reared on a wheat germ based diet.
Trialeurodes vaporariorum: The stock culture was maintained on eggplant seedlings (Solanum melongena L.) reared in a glasshouse under controlled conditions of 25 ± 1°C, 70 ± 10% RH.
Tetranychus urticae: The stock culture was maintained on sweetpotato plants (Ipomoea batatas [L.] Lam.) in a glasshouse under controlled conditions of 25 ± 1°C, 70 ± 10% RH.
Gynaikothrips ficorum: Large populations of G. ficorum infest Ficus trees (Ficus nitida Thunb.) in Egypt, causing immense damage (Tawfik 1967; Ragab 1991). Thus, large numbers of larvae of G. ficorum were collected directly from Ficus trees cultivated on the Experimental farm for use in experiments. Second instars were used in all experiments.
Effect of different prey on the immature stages of O. albidipennis
The effect of prey type on nymphal development, survival and predation was determined at 26 ± 1°C, 60 ± 10% RH and 16L:8D photoperiod. Newly eclosed nymphs (0–6 h old) were separated individually into small petri dishes (9 cm diameter × 1.5 cm height) by using a small fine-hair brush. There were 47–49 replicates for each prey treatment.
Each nymph was provided with one of the four investigated prey (eggs of E. kuehniella, T. urticae, T. vaporariorum, or 2nd instars of G. ficorum). Eggs of E. kuehniella were glued onto pieces of filter paper with a diluted solution of Arabic gum (Tommasini et al. 2004) and placed on moistened filter paper discs, which provided additional moisture in the dishes. The other prey were provided to the predators on small leaf discs cut from host plants used in their colony’s maintenance.
Each container was inspected daily to determine O. albidipennis development stage and the number of prey consumed. Predation was determined by visual examination of prey under a binocular microscope (20×), after removal from the petri dish arenas. New prey were provided on a daily basis until the O. albidipennis completed development or died, according to the schedule shown in Table 1. The amount of prey provided was based on preliminary experiments to ensure that more prey were available to predators than they would consume within one day. Therefore, immature development would not be affected by prey limitation.
Data were recorded in the form of developmental time, mortality, and prey consumption for each instar (El-Husseini et al. 1993). After adult eclosion, the sex ratio of O. albidipennis reared on each of the four prey types was determined and expressed as percent female.
Effect of different prey on the longevity and female fecundity of O. albidipennis
Fecundity and longevity were determined for adults emerging from the immature development tests. Newly emerged adults of O. albidipennis for the four prey treatments were paired (one female with one male), and these pairs were placed separately in petri dishes (9 cm diameter × 1.5 cm height) for copulation. To stimulate mating, no prey were added at this time (Ortigosa and Rowe 2002). Twelve hours later, males were removed and separated to other petri dishes so that responses of individual predators could be determined.
The females were supplied daily with fresh excess prey on new plant leaves or paper discs, and bean pods as oviposition sites until death. In a manner similar to females, males were also provided with fresh excess prey, and bean pods, as Orius spp. will obtain moisture and nutrients from feeding on plant material (Salas-Aguilar and Ehler 1977). The number of consumed prey and deposited eggs were counted daily, under a binocular microscope (20X). Experiments were conducted under the same laboratory conditions as for the experiment with nymphs (26 ± 1°C, 60 ± 10% RH and 16L:8D photoperiod).
Statistical analyses
The data on nymph development and nymph prey consumption were analyzed by one-way ANOVA’s, with Tukey’s post-hoc comparison of treatment means. If assumptions for normally distributed data with homogeneous variances could not be fulfilled, we used the non-parametric Kruskal–Wallis ANOVA on ranks and compared treatment effects using Dunn’s test. These analyses were performed with Sigmastat 2.03 (SPSS Inc, Chicago, IL, USA). Survival data and sex ratios were examined with a generalized linear model (GLM) fitted by maximum quasi-binomial estimation in the software package R (R: A Language and Environment for Statistical Computing, version 2.10.1, Zurich, Switzerland, 2009, http://www.R-project.org).
Adult prey consumption and adult longevity were analyzed by two-way ANOVA, with diet type and predator sex as treatment factors. In the event of a significant interaction, separate one-way ANOVA’s for diet type were conducted for each sex. If assumptions for normally distributed data with homogeneous variances could not be fulfilled, we used the non-parametric Kruskal–Wallis ANOVA on ranks and compared treatment effects using Dunn’s test. Female fecundity was analyzed using one-way ANOVA and Tukey’s post-hoc comparison of treatment means when the data were normally distributed and the variances were homogeneous. These analyses were performed with Sigmastat 2.03.
Life table parameters were estimated for O. albidipennis fed on each prey diet. Parameter estimates and their 95% confidence limits were calculated for the net reproductive rate (R0), the mean generation time (T), the doubling time (DT), the intrinsic rate of natural increase (r m ), and the finite capacity of increase (λ), using the jackknife technique developed by Maia et al. (2000). This technique allows for statistical comparisons to be made among the parameters for the different prey diet treatments. Data for the egg stage used in these calculations were obtained from Sobhy et al. (2006) for similar environmental conditions.
Results
Effect of different prey on the immature stages of O. albidipennis
As shown in Table 2, the type of prey had profound effects on the duration of all nymphal instars of O. albidipennis. Total developmental time was significantly faster for O. albidipennis that fed on E. kuehniella eggs or G. ficorum larvae compared with the other investigated prey (F = 175.822; df = 3,126; P < 0.001). Significant differences were found in development time for all instars O. albidipennis among the different prey diets (first instar: H = 54.083; df = 3; P < 0.001; second: H = 105.410; df = 3; P < 0.001; third: H = 31.709; df = 3; P < 0.001; fourth: H = 77.004; df = 3; P < 0.001; and fifth: H = 93.102; df = 3; P < 0.001), with proportionately greater differences in development time occurring in the later stadia.
Orius albidipennis nymphs were able to complete their development on all tested diets, although there were significant differences in survivorship (χ2 = 18.90, df = 3, P = 0.004; Table 2). The lowest overall survival rates to adulthood were recorded for nymphs that fed on T. vaporariorum eggs (51.06%) and on T. urticae eggs (59.57%), while the highest survival rate (87.75%) was recorded for those fed on E. kuehniella eggs. Intermediate rates for survivorship were reported for those fed on G. ficorum (72.91%) (Table 2).
The type of prey also had a significant effect on the number of prey consumed by O. albidipennis nymphs (H = 124.540; df = 3; P < 0.001 for the 1st instar; H = 116.103; df = 3; P < 0.001 for the 2nd instar; H = 115.231; df = 3; P < 0.001 for the 3rd instar; H = 107.952; df = 3; P < 0.001 for the 4th instar and F = 857.467; df = 3,126; P < 0.001 for the 5th instar). The prey consumption increased notably as O. albidipennis aged. The greatest total prey consumption was 209.85 eggs of T. urticae and the lowest was 62.25 eggs of E. kuehniella (Fig 1).
Prey consumption (Means + SE) of different nymphal stages of Orius albidipennis fed one of four different prey (Ephestia kuehniella; Gynaikothrips ficorum; Tetranychus urticae; Trialeurodes vaporariorum). For each instar, different letters above the bars indicate a significant difference between tested prey (P < 0.05), based on a post-hoc Dunn’s test
However, because of the differences in development times for predators reared on the different prey, the mean daily predation rates (prey/day) were higher with G. ficorum as the prey than for the other types of prey, especially for the younger instars of O. albidipennis.
Females outnumbered males, with slightly female biased sex ratios occurring in all treatments, although these were not significantly different (Table 2). The lowest proportions of females occurred among O. albidipennis reared on eggs of T. urticae and T. vaporariorum, whereas the highest proportion of females was 62.85% when G. ficorum larvae were supplied as prey.
Effect of different prey on the longevity and fecundity of O. albidipennis
Longevity of adult O. albidipennis was dependent on both prey type and predator sex, with females living considerably longer than males. There was a significant interaction between prey type and predator sex (F = 14.53; df = 3,122; P < 0.0001), which was a result of the comparatively short longevity of males fed E. kuehniella eggs. Because of the significant interaction, separate analyses were conducted for females and males. Significant differences in adult longevity were observed among the different prey diets (H = 42.834; df = 3; P < 0.001 for females; H = 39.191; df = 3; P < 0.001 for males). The shortest longevity for females (14.33 days) was for those fed T. urticae eggs whereas the longest (26.86 days) was recorded for those fed G. ficorum larvae. The longest longevity for males was for those fed G. ficorum larvae (8.76 days), and the shortest longevity (3.97 days) was for those fed E. kuehniella eggs.
Prey species had a significant effect on the fecundity of O. albidipennis. (F = 534.991; df = 3,71; P < 0.001). The greatest lifetime fecundity (147.88 eggs/female) was recorded for females fed E. kuehniella eggs (Table 3). When females fed on G. ficorum larvae, fecundity dropped to 93.36 eggs/female, but fecundity was even significantly lower for females fed eggs of T. urticae or eggs of T. vaporariorum. Females on those diets produced only 42.77–36.67% of the eggs, respectively, produced by females that fed on E. kuehniella eggs.
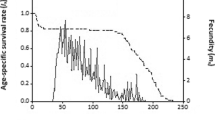
The greatest mean daily fecundity, averaged over female lifetime, (7.71 eggs/female/day) was also recorded for females that were fed E. kuehniella eggs (Table 3). Females fed the other prey diets produced only 37.35–54.73% of the eggs produced daily by females fed E. kuehniella eggs (H = 66.827; df = 3; P < 0.001). Oviposition was not constant over time. Oviposition patterns for each prey type showed an initial increase early in adulthood followed by declines as females aged. Although oviposition declined over time, most females were able to continue egg laying almost until death (Fig. 2). Peak daily oviposition occurred on day 7 of adulthood for females fed E. kuehniella eggs, with these females laying 15.64 ± 1.08 eggs (mean ± SE). This amount was over twice the maximum daily oviposition recorded for females reared on the other prey types (6.50 ± 0.40) eggs laid on day 10 for G. ficorum as prey; 7.07 ± 0.41 eggs laid on day 6 for T. urticae eggs as prey; 5.00 ± 0.23 eggs laid on day 8 for T. vaporariorum eggs as prey).
Prey type had a significant effect on prey consumption by O. albidipennis adults. There was a significant prey type by predator sex interaction for total prey consumption (F = 21.74; df = 3,122; P < 0.0001), but overall, female predators did consume significantly more total prey than male predators (F = 3677; df = 1,122; P < 0.0001). Part of this difference is attributable to differences in longevity between males and females. Although females did consume more eggs of E. kuehniella or T. urticae per day than did corresponding males, daily predation rates were similar between males and females fed either G. ficorum or T. vaporariorum. These differences produced a significant interaction for predator sex by prey type (F = 8.52; df = 3,122; P < 0.0001; Table 3).
When considering the sexes separately, O. albidipennis females differed significantly in their lifetime consumption of the four tested prey (F = 31.143; df = 3,71; P < 0.001). Lifetime prey consumption was greatest when females were given T. urticae eggs (127.53) or E. kuehniella eggs (123.96) and lowest (98.23) when given T. vaporariorum eggs (Table 3).
When accounting for differences in longevity, females consumed more T. urticae eggs per day than other types of prey. Significant differences also existed among the tested prey in lifetime prey consumption by male O. albidipennis (F = 32.204; df = 3,51; P < 0.001). The lowest daily prey consumption was for males given G. ficorum larvae as prey, although these males did have the greatest mean longevity (Table 3).
In terms of the number of eggs females laid per prey item, E. kuehniella fed females produce over 1.2 eggs per prey item. Females fed G. ficorum produced 0.8 eggs per prey item. In contrast, females fed either fed T. urticae or T. vaporariorum produced less than 50% of the eggs per prey item that females fed E. kuehniella did.
Life table parameters
Life table parameter estimates (Table 4) reinforce that E. kuehniella eggs were a superior diet for O. albidipennis compared with the other prey tested. Because of the rapid nymphal development time and high fecundity early in adulthood, O. albidipennis fed E. kuehniella eggs had a significantly greater net reproductive rate (Ro), intrinsic rate of natural increase (r m ), and finite rate of increase (λ) than those fed other prey (Table 4). The mean generation time and population doubling time were also significantly shorter when E. kuehniella eggs were prey compared with other prey types. Although O. albidipennis reared on G. ficorum larvae had a rapid development, the length of time to reach their peak oviposition resulted in relatively long generation and doubling times.
Discussion
Although generalist predatory insects are capable of attacking a diverse spectrum of prey species, the results of our study show that the type of prey can substantially impact a wide range of characteristics related to the performance of O. albidipennis, including development, survival and fecundity, which have important implications for population dynamics. Because the quality of food for predatory insects can be measured by growth and oviposition (Arijs and De Clercq 2004), it is important to consider effects of prey across both immature and adult stages. Our results from rearing O. albidipennis from neonate first instars until adult death on specific diets indicate that eggs of E. kuehniella were the most suitable for rearing O. albidipennis among the different arthropod prey tested. These results are in agreement with studies of other species of Orius (Kiman and Yeargan 1985; Bush et al. 1993; Richards and Schmidt 1996; Nagai et al. 1998; Mendes et al. 2002; Bonte and De Clercq 2008). However, some comparative studies have found that thrips, including G. ficorum, are more suitable than other arthropod prey, including Lepidoptera eggs for O. albidipennis (Tawfik and Ata 1973; Chyzik et al. 1995b). Thrips are generally considered a key component of natural diets of Orius spp. (Lattin 1999), and Orius spp. typically demonstrate a preference for thrips over other types of prey in choice situations (Kakimoto et al. 2006; Arnò et al. 2008; Xu and Enkegaard 2009).
Although O. albidipennis nymphs completed their development on all of the four tested prey, not all of the prey were equally suitable for development. Nymphal development time was shortest and survivorship was greatest when E. kuehniella eggs were provided as prey. Nymphs given E. kuehniella eggs had relatively high survivorship throughout all stadia (>95% survived each stadium). Mortality was highest for 4th instars reared on G. ficorum larvae. For predators reared on eggs of T. urticae or T. vaporariorum, the greatest mortality occurred during the first stadium. These results are similar to most other studies comparing the development of Orius spp. on Lepidoptera eggs versus thrips (Kiman and Yeargan 1985; Nagai et al 1998; Richards and Schmidt 1996), in which developmental rates and survival were lower with thrips as prey. In one notable exception, Mendes et al. (2002) found that development of O. insidiosus was longer with eggs of E. kuehniella as prey than with adults of Caliothrips phaseoli (Hood) as prey.
The order of suitability of the different prey for adults was similar to the order of suitabilities for nymphs. Female O. albidipennis had the highest fecundity when fed eggs of E. kuehniella. Although thrips larvae are considered a preferred prey for Orius spp. (Nagai 1991; Yano 1996; Kohono and Kashio 1998; Mendes et al. 2002; Baez et al. 2004), G. ficorum larvae were not the most suitable prey for O. albidipennis in our study. Larvae of G. ficorum were a more suitable prey than eggs of T. urticae or T. vaporariorum, which produced females with significantly shorter longevities and lower fecundities. Yet, despite longevity being the longest for females fed G. ficorum larvae, these females had much lower reproductive rates and lifetime fecundity than those fed eggs of E. kuehniella. This increase in fecundity with E. kuehniella eggs as prey was not just a reflection of differences in longevity of O. albidipennis, as daily oviposition was 1.8–2.6 times higher for females fed on E. kuehniella than on other prey diets. Total fecundity was 1.6–2.7 times higher with E. kuehniella than with the other prey. Interestingly, although we found that it took relatively few eggs of E. kuehniella for O. albidipennis to complete development, adult female predators consumed as many or more E. kuehniella eggs as any other prey.
Eggs of T. urticae and T. vaporariorum may be poor quality prey because they lack certain essential nutrients, which may slow development, increase mortality, or hinder ovigeny. Because we gave immature and adult O. albidipennis an excess of fresh prey each day, the differential response to prey type is likely the result of differences in the nutritional value of the prey rather than a scarcity of food or differences in searching times. Hagler et al. (2004) concluded that sweetpotato whitefly, Bemisia tabaci (Gennadius), eggs are a non preferred prey for O. tristicolor (White). Arnò et al. (2008) found that the different life stages of B. tabaci could support development of Orius majusculus (Reuter) and O. laevigatus, but these predators ultimately preferred the thrips F. occidentalis over B. tabaci. For all instars of O. albidipennis fed on eggs of T. urticae, there was a tendency towards an increased development time and relatively low survival rates. Paik et al. (2003) found that O. sauteri (Poppius) was also able to complete development on eggs of T. urticae, but this prey resulted in slower development and lower survivorship than prey such as thrips larvae.
The high quality of Lepidoptera eggs is likely related to their relatively high nitrogen content. Ferkovich et al. (2007) found that E. kuehniella eggs had the highest protein concentrations among five Lepidoptera species tested. It is also possible that the fat content is higher in E. kuehniella eggs than in other prey, which could contribute to the greater development rate and reproduction on that diet (De Clercq et al 2005). Indeed, Specty et al. (2003) found that E. kuehniella eggs have two times the amino acid content and three times greater lipid content than the pea aphid Acyrthosiphon pisum (Harris), and that these differences were related to variation in the size and fecundity of the coccinellid predator Harmonia axyridis Pallas reared on those prey. Lepidopteran eggs may also contain additional factors that enhance reproduction in Orius species (Ferkovich and Shapiro 2004).
Compared with other species of Orius, few studies have examined the potential of O. albidipennis as a biological control agent. However, it is among the most common species of Orius in much of its native range (Tawfik and Ata 1973; Hernandez and Stonedahl 1999) where it would be suitable for augmentative biological control (van Lenteren et al. 2003; Louda et al. 2003). The results of our studies provide certain basic biological data on O. albidipennis and its potential impact on various prey species in the field. Data on predation rates and population growth are important for forecasting the role of O. albidipennis as a biological control agent. Although laboratory based data need to be corroborated with field studies, our results show that O. albidipennis can survive and reproduce successfully on alternative types of prey in the absence of more favourable types of prey. Furthermore, based on findings for O. insidiosus (Harwood et al. 2007), it is likely that O. albidipennis would switch among available prey types in the field. This exemplifies one highly favourable trait of Orius species as biological control agents, that they are able to persist when target prey are scarce (Hassell and Rogers 1972). While this trait is often cited as making Orius spp. a good biocontrol agent, differences in prey quality need to be considered to better understand predator–prey dynamics in the field (Sabelis and van Rijn 1997; Vacante et al. 1997). For example, predation on poor quality prey would slow down predator population growth and reduce the ability of predator populations to control alternative herbivore pests.
In addition to the ecological relevance of our findings, they also can serve to assist with the development of mass rearing of predators in developing countries such as Egypt where the dynamics of biological control production practices may differ from those in developed countries (Altieri et al. 1997; Silveira et al. 2004). While our results indicate that E. kuehniella is the most suitable diet for O. albidipennis, G. ficorum larvae can be a suitable prey. Further, T. urticae eggs and T. vaporariorum eggs could be used as alternative diets in maintaining O. albidipennis if more suitable prey are not readily available.
References
Altieri MA, Rosset PM, Nicholls CI (1997) Biological control and agricultural modernization: towards resolution of some contradictions. Agric Human Values 14:303–310
Arijs Y, De Clercq P (2004) Liver-based artificial diets for the production of Orius laevigatus. BioControl 49:505–516
Arnò J, Roig J, Riudavets J (2008) Evaluation of Orius majusculus and O. laevigatus as predators of Bemisia tabaci and estimation of their prey preference. Biol Control 44:1–6
Baez I, Reitz SR, Funderburk JE (2004) Predation by Orius insidiosus (Heteroptera: Anthocoridae) on species and life stages of Frankliniella flower thrips (Thysanoptera: Thripidae) in pepper flowers. Environ Entomol 33:662–670
Bonte M, De Clercq P (2008) Developmental and reproductive fitness of Orius laevigatus (Hemiptera: Anthocoridae) reared on factitious and artificial diets. J Econ Entomol 101:1127–1133
Bush L, Kring TJ, Ruberson JR (1993) Suitability of greenbugs, cotton aphids and Heliothis virescens eggs for development and reproduction of Orius insidiosus. Entomol Exp Appl 67:217–222
Butler CD, O’Neil RJ (2007) Life history characteristics of Orius insidiosus (Say) fed Aphis glycines Matsumura. Biol Control 40:333–338
Chambers RJ, Long S, Helyer NL (1993) Effectiveness of Orius laevigatus (Hem, Anthocoridae) for the control of Frankliniella occidentalis on cucumber and pepper in the UK. Biocontrol Sci Technol 3:295–307
Chyzik R, Ucko O (2002) Seasonal abundance of the Western Flower Thrips Frankliniella occidentalis in the Arava Valley of Israel. Phytoparasitica 30:335–346
Chyzik R, Klein M, Ben-Dov Y (1995a) Overwintering biology of the predatory bug Orius albidipennis (Hemiptera: Anthocoridae) in Israel. Biocontrol Sci Technol 5:287–296
Chyzik R, Klein M, Ben-Dov Y (1995b) Reproduction and survival of the predatory bug Orius albidipennis on various arthropod prey. Entomol Exp Appl 75:27–31
Cocuzza GE, De Clercq P, Lizzio S, Van de Veire M, Tirry L, Degheele D, Vacante V (1997a) Life tables and predation activity of Orius laevigatus and O. albidipennis at three constant temperatures. Entomol Exp Appl 85:189–198
Cocuzza GE, De Clercq P, Van de Veire M, De Cock A, Degheele D, Vacante V (1997b) Reproduction of Orius laevigatus and Orius albidipennis on pollen and Ephestia kuehniella eggs. Entomol Exp Appl 82:101–104
Coll M, Shakya S, Shouster I, Nenner Y, Steinberg S (2007) Decision-making tools for Frankliniella occidentalis management in strawberry: consideration of target markets. Entomol Exp Appl 122:59–67
Cranshaw W, Sclar DC, Cooper D (1996) A review of 1994 pricing and marketing by suppliers of organism for biological control of arthropods in the United States. Biol Control 6:291–296
De Clercq P, Arijs Y, Van Meir T, Van Stappen G, Sorgeloos P, Dewettinck K, Rey M, Grenier S, Febvay G (2005) Nutritional value of brine shrimp cysts as a factitious food for Orius laevigatus (Heteroptera: Anthocoridae). Biocontrol Sci Technol 15:467–479
Dissevelt M, Altena K, Ravensberg WJ (1995) Comparison of different Orius species for control of Frankliniella occidentalis in glasshouse vegetable crops in the Netherlands. Meded Fac Landbouwk Toeg Biol Wet Univ Gent 60:839–845
El-Husseini M, Schumann K, Sermann H (1993) Rearing immature feeding stage of Orius majusculus Reut. on the acarid mite Tyrophagus putrescentiae Schr. as a new alternative prey. J Appl Entomol 116:113–117
Eubanks MD, Denno RF (2000) Health food versus fast food: the effects of prey quality and mobility on prey selection by a generalist predator and indirect interactions among prey species. Ecol Entomol 25:140–146
Ferkovich SM, Shapiro JP (2004) Comparison of prey-derived and non-insect supplements on egg-laying of Orius insidiosus maintained on artificial diet as adults. Biol Control 31:57–64
Ferkovich SM, Venkatesan T, Shapiro JP, Carpenter JE (2007) Presentation of artificial diet: effects of composition and size of prey and diet domes on egg production by Orius insidiosus (Heteroptera: Anthocoridae). Fla Entomol 90:502–508
Fritsche ME, Tamò M (2000) Influence of thrips prey species on the life history and behaviour of Orius albidipennis. Entomol Exp Appl 96:111–118
Funderburk J (2009) Management of the western flower thrips (Thysanoptera: Thripidae) in fruiting vegetables. Fla Entomol 92:1–6
Hagler JR, Jackson CG, Machtley SA, Isaacs R (2004) Foraging behavior and prey interactions by a guild of predators on various lifestages of Bemisia tabaci. J Insect Sci 4:1–13
Harwood JD, Desneux N, Yoo HJS, Rowley DL, Greenstone MH, Obrycki JJ, O’Neil RJ (2007) Tracking the role of alternative prey in soybean aphid predation by Orius insidiosus: a molecular approach. Mol Ecol 16:4390–4400
Hassell MP, Rogers DJ (1972) Insect parasite responses in the development of population models. J Anim Ecol 42:661–676
Hernandez LM, Stonedahl GM (1999) A review of the economically important species of the genus Orius (Heteroptera: Anthocoridae) in East Africa. J Nat Hist 33:543–568
Isenhour DJ, Yeargan KV (1981) Predation by Orius insidiosus on the soybean thrips, Sericothrips variabilis: effect of prey stage and density. Environ Entomol 10:496–500
Kakimoto K, Inoue H, Hinomoto N, Noda T, Hirano K, Kashio T, Kusigemati K, Okajima S (2006) Potential of Haplothrips brevitubus (Karny) (Thysanoptera: Phlaeothripidae) as a predator of mulberry thrips Pseudodendrothrips mori (Niwa) (Thysanoptera: Thripidae). Biol Control 37:314–319
Kiman ZB, Yeargan KV (1985) Development and reproduction of the predator Orius insidiosus (Hemiptera: Anthocoridae) reared on diets of selected plant material and arthropod prey. Ann Entomol Soc Am 78:464–467
Kohono K, Kashio T (1998) Development and prey consumption of Orius sauteri (Poppius) and Orius minutus (L.) (Heteroptera: Anthocoridae) fed on Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Appl Entomol Zool 33:227–230
Lattin JD (1999) Bionomics of the Anthocoridae. Annu Rev Entomol 44:207–231
Lee GH, Choi MY, Kim DH (1996) Predatory characteristic of Orius sauteri on two prey species of Myzus persicae and Tetranychus urticae. RDA J Agric Sci Crop 38:501–506
Louda SM, Pemberton RW, Johnson MT, Follett PA (2003) Nontarget effects—the Achilles’ heel of biological control? Retrospective analyses to reduce risk associated with biocontrol introductions. Annu Rev Entomol 48:365–396
Maia A, Luiz A, Campanhola C (2000) Statistical inference on associated fertility life table parameters using Jackknife technique: computational aspects. J Econ Entomol 93:511–518
Mendes SM, Bueno VHP, Argolo VM, Silveira LCP (2002) Type of prey influences biology and consumption rate of Orius insidiosus (Say) (Hemiptera: Anthocoridae). Rev Bras Entomol 46:99–103
Nagai K (1991) Predatory characteristics of Orius sp. on Thrips palmi Karny Tetranychus kanzawai Kishida and Aphis gossypii Clover. Jpn J Appl Entomol Zool 35:283–290
Nagai K, Hirose Y, Takagi M, Nakashima Y, Hiramatsu T (1998) Selection of alternative prey for rearing Orius tantillus (Motschulsky). Jpn J Appl Entomol Zool 42:85–87
Ortigosa A, Rowe L (2002) The effect of hunger on mating behaviour and sexual selection for male body size in Gerris buenoi. Anim Behav 64:369–375
Paik CH, Hwang CY, Lee GH, Kim DH, Choi-Man Y, Na SY, Kim SS (2003) Development, reproduction and longevity of predator Orius sauteri (Poppius) (Hemiptera: Anthocoridae) when reared on three different preys. Korean J Appl Entomol 42:35–41
Perdikis D, Kapaxidi E, Papadoulis G (2008) Biological control of insect and mite pests in greenhouse Solanaceous crops. Eur J Plant Sci Biotechnol 2:125–144
Ragab ME (1991) Entomophagous insects associated with Gynaikothrips ficorum Marchal on Ficus nitida Thumb trees in Mansoura. Egypt J Biol Pest Control 1:59–67
Reitz SR, Funderburk JE, Waring SM (2006) Differential predation by the generalist predator Orius insidiosus on congeneric species of thrips that vary in size and behaviour. Entomol Exp Appl 119:179–188
Richards PC, Schmidt JM (1996) The effects of selected dietary supplements on survival and reproduction of Orius insidiosus (Say). Can Entomol 128:171–176
Riudavets J (1995) Predators of Frankliniella occidentalis (Per.) and Thrips tabaci Lind.: a review. Wageningen Agric Univ Pap 95:43–87
Sabelis MW, van Rijn PCJ (1997) Predation by mites and insects. In: Lewis T (ed) Thrips as crop pests. CAB International, Wallingford, UK, pp 259–354
Salas-Aguilar J, Ehler LE (1977) Feeding habits of Orius tristicolor. Ann Entomol Soc Am 70:60–62
Salim M, Masud SA, Khan AM (1987) Orius albidipennis (Reut.) (Hemiptera: Anthocoridae), a predator of cotton pests. Philipp Entomol 7:37–42
Sanchez JA, Lacasa A (2002) Modelling population dynamics of Orius laevigatus and O. albidipennis (Hemiptera: Anthocoridae) to optimize their use as biological control agents of Frankliniella occidentalis (Thysanoptera: Thripidae). Bull Entomol Res 92:77–88
Silveira LCP, Bueno VHP, van Lenteren JC (2004) Orius insidiosus as biological control agent of thrips in greenhouse chrysanthemums in the tropics. Bull Insectol 57:103–109
Sobhy IS, Sarhan AA, Shoukery AA, El-Kady GA, Mandour NS (2005) Effect of different types of ovipositional substrates and shelters on the mass rearing parameters of Orius albidipennis (Reuter) (Hemiptera: Anthocoridae). J Agric Res Suez Canal Univ 5:115–118
Sobhy IS, Sarhan AA, Shoukery AA, El-Kady GA, Mandour NS (2006) Effects of different temperature levels on the biological attributes of Orius albidipennis (Reuter) (Hemiptera: Anthocoridae). J Agric Res Suez Canal Univ 6:213–216
Specty O, Febvay G, Grenier S, Delobel B, Piotte C, Pageaux J-F, Ferran A, Guillaud J (2003) Nutritional plasticity of the predatory ladybeetle Harmonia axyridis (Coleoptera: Coccinellidae): Comparison between natural and substitution prey. Arch Insect Biochem Physiol 52:81–91
Tawfik MSF (1967) Microfauna of leaf-rolled Ficus nitida Thumb. Bull Entomol Soc Egypt 51:483–487
Tawfik MFS, Ata AM (1973) The life history of Orius albidipennis (Reut.) (Hemiptera: Anthocoridae). Bull Entomol Soc Egypt 57:117–126
Tommasini MG, van Lenteren JC, Burgio G (2004) Biological traits and predation capacity of four Orius species on two prey species. Bull Insectol 57:79–93
Vacante V, Cocuzza GE, De Clercq P, Van De Veire M, Tirry L (1997) Development and survival of Orius albidipennis and O. laevigatus (Het.: Anthocoridae) on various diets. Entomophaga 42:493–498
van den Meiracker RAF (1994) Induction and termination of diapause in Orius predatory bugs. Entomol Exp Appl 73:127–137
van den Meiracker RAF, Ramakers PMJ (1991) Biological control of the western flower thrips Frankliniella occidentalis, in sweet pepper with the anthocorid predator Orius insidiosus. Meded Fac Landbouww Rijksuniv Gent 56:241–249
van Lenteren JC, Roskam MM, Timmer R (1997) Commercial mass production and pricing of organisms for biological control of pests in Europe. Biol Control 10:143–149
van Lenteren JC, Babendreier D, Bigler F, Burgio G, Hokkanen HMT, Kuske S, Loomans AJM I, Menzler-Hokkanen I, van Rijn PCJ, Thomas MB, Tommasini MG, Zeng QQ (2003) Environmental risk assessment of exotic natural enemies used in inundative biological control. BioControl 48:3–38
Xu X, Enkegaard A (2009) Prey preference of Orius sauteri between Western Flower Thrips and spider mites. Entomol Exp Appl 132:93–98
Yano E (1996) Biology of Orius sauteri (Poppius) and its potential as a biological agent for Thrips palmi Karny. IOBC/WPRS Bull 19:203–206
Zaki FN (1989) Rearing of two predators. Orius albidipennis (Reut.) and Orius laevigatus (Fieber) on some insect larvae. J Appl Entomol 107:107–109
Acknowledgments
The authors would like to thank all of the technicians of the Public Service Centre of Biological Control (PSCBC), Faculty of Agriculture, Suez Canal University, Ismailia, Egypt for their assistance and the supplement of E. kuehniella eggs. We particularly thank Prof. Dr. Awadallah, K. T., Faculty of Agriculture, Cairo University, Egypt, for his help in the identification of Orius albidipennis individuals, which were used to build up the mother colony. We acknowledge the comments of Heather Murray, Zürich University and Jeremy McNeil, University of Western Ontario on an earlier draft of the manuscript. We are grateful to Matthias Held and Georg von Mérey for statistical advice; Matthias Erb, Ted Turlings, FARCE Lab., Institute of Biology, Neuchâtel University, and Eric Riddick and Jeff Shapiro, USDA-ARS for their helpful suggestions on the manuscript. Our Sincere thanks for Dr. Patrick De Clercq and the Handling Editor for their valuable contributions and constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Patrick De Clercq.
Rights and permissions
About this article
Cite this article
Sobhy, I.S., Sarhan, A.A., Shoukry, A.A. et al. Development, consumption rates and reproductive biology of Orius albidipennis reared on various prey. BioControl 55, 753–765 (2010). https://doi.org/10.1007/s10526-010-9304-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-010-9304-z