Abstract
Harmonia axyridis Pallas (Coleoptera: Coccinellidae) is native to Asia, and was widely introduced as a biocontrol agent of aphids and coccids in Europe and North America. In Europe, H. axyridis is considered to be an invasive alien species because of its potential to disrupt native ladybird communities. Since 1999, the Belgian Ladybird Working Group mapped all Belgian Coccinellidae and recorded data on substratum plants and habitat. The first feral H. axyridis population in Belgium was recorded in 2001, but the expansion rate is decreasing because it now colonised the whole country. Recorded occupancy in Belgium showed an average rate of increase of 189% between 2002 and 2006. In Belgium, H. axyridis occurred in a wide range of habitats, including those of high conservation value. However, habitat and land cover analysis showed that H. axyridis is more frequently found in urbanised landscapes than in semi-natural landscapes. A niche overlap analysis based on plant use data showed that the potential to affect native species is higher for generalist, deciduous and coniferous tree ladybird species than for heathland and wetland specialist species. Phenology data showed that H. axyridis is able to reproduce later in the year than native species. Based on recorded distribution, ecology and phenology, we discuss the potential of H. axyridis to disrupt native ladybird assemblages in Belgium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Harmonia axyridis Pallas (Coleoptera: Coccinellidae) is used as a biocontrol agent against aphid populations because its larvae are very voracious, polyphagous and easy to rear (Ferran et al. 1996; Maignet 2002; Koch 2003). The species has been widely used in greenhouses, orchards and gardens in North America since 1916 (Gordon 1985) and in Western Europe since 1982 (Iperti and Bertrand 2001), where it was sold by different private companies (Adriaens et al. 2003; Poutsma et al. 2007). Harmonia axyridis, among other predatory insects and parasitoids, was ranked as a high-risk species in an environmental risk assessment of exotic natural enemies used in inundative biological control (van Lenteren et al. 2003; van Lenteren et al. 2007). Harmonia axyridis is known to have strong dispersal capacities (Koch 2003; Osawa 2000) and studies in North America have shown that it can rapidly colonise large areas (Tedders and Schaefer 1994). In Europe, it has spread very rapidly, particularly since 2002, and the species now exists as feral populations in 13 European countries (Brown et al. 2007a).
Laboratory experiments showed that H. axyridis is frequently involved in intraguild interactions with other aphidophagous species, such as the ladybird species Adalia bipunctata (L.) and Coccinella septempunctata L. (Coleoptera: Coccinellidae), both native to Western Europe (Hironori and Katsuhiro 1997; Cottrell and Yeargan 1998; Yasuda and Ohnuma 1999; Yasuda et al. 2001). Petri dish experiments showed that 4th instar H. axyridis larvae easily attack A. bipunctata larvae in the absence of other prey (Hautier 2003a, b, 2006). Furthermore, H. axyridis larvae showed aggressive behaviour towards the first three larval stages of A. bipunctata, resulting in over 80% mortality after 30 min and 100% mortality for all three larval stages after 24 h (Hautier 2003a). In laboratory experiments, the presence of aphids as alternative prey did not alter the mortality figures of A. bipunctata. These results showed that A. bipunctata is a potential prey for H. axyridis under limited food conditions. Therefore, H. axyridis can be regarded as an important source of mortality for native ladybird species. Moreover, native species rarely consumed eggs and larvae of H. axyridis. Observations in potato fields showed that oviposition of H. axyridis usually occur late during aphid development and is potentially accompanied by strong intraguild predation against native ladybird species (Hautier 2006). It was shown that H. axyridis can reproduce on a pollen diet, when insect prey is scarce, which gives the species a competitive advantage over indigenous predatory ladybird species that are less capable of doing so (Berkvens et al. 2007). However, there are indications that A. bipunctata is also able to compensate for a suboptimal diet of animal prey by supplementary feeding on flower pollen (De Clercq et al. 2005).
Harmonia axyridis can induce dominance shifts in ladybird communities and often becomes the predominant species at the expense of native species (Lamana and Miller 1996; Brown and Miller 1998; Colunga-Garcia and Gage 1998; Burgio et al. 2002; Michaud 2002, 2004; Alyokhin and Sewell 2004). In Belgium, modifications in ladybird communities have been reported through detailed monitoring studies in Brussels (San Martin 2003; Ottart 2005). Prior to the invasion of H. axyridis, A. bipunctata was the dominant lady beetle on lime (Tilia sp.) and maple (Acer sp.) in this urban environment and was co-dominant with the native congeneric ladybird Harmonia quadripunctata (Pontoppidan) (Coleoptera: Coccinellidae) on Austrian pine (Pinus nigra Arn.). Only 2 years after its establishment, H. axyridis quickly became the predominant ladybird species in all habitats monitored (Ottart 2005). In addition, a significant decline in the abundance of two native species A. bipunctata and Adalia decempunctata (L.) (Coleoptera: Coccinellidae) was recorded between 2003 and 2005, while a simultaneous increase of the H. axyridis population was observed (Ottart 2005).
Due to its voracity and wide trophic niche, it was believed that H. axyridis would harm native aphidophagous guilds. Despite the relatively large body of knowledge on H. axyridis, our knowledge of the potential adverse impacts and other non-target effects needs to be improved (Koch 2003). Studies have shown declines in densities of native ladybird species after the arrival of H. axyridis, e.g. in orchards (Tedders and Schaefer 1994), potato fields (Alyokhin and Sewell 2004) and other agricultural ecosystems (Colunga-Garcia and Gage 1998). However, very few articles deal with H. axyridis population dynamics in (semi)-natural ecosystems.
The knowledge of processes and factors explaining the invasion success is still rudimentary (Williamson 2006). The recent establishment of H. axyridis in Belgium and elsewhere in Europe provides a unique opportunity to study invasion mechanisms and the impact of an exotic predator on native organisms. Explaining and predicting the success of an invasive species like H. axyridis requires information on microhabitat and phenology.
In this article, we analyse distributional and phenological data collected during a large-scale mapping scheme in Belgium to detect whether H. axyridis is able to modify native ladybird communities. First, we investigated differences in habitat selection between H. axyridis and native species. Second, we analysed the occurrence of H. axyridis in areas of conservation concern, using biological valuation and land cover maps. Finally, we discuss the potential impact on native species based on a niche overlap analysis.
Material and methods
The Belgian ladybird mapping project
In 1999, the Belgian ladybird working group Coccinula launched a large-scale field survey on 40 native ladybird species (Coccinellinae, Chilocorinae and Epilachninae) and to date has 527 volunteers providing distribution data. They actively search for ladybirds in a variety of habitats using sweep nets, beating trays, visual search, light trapping, pitfall traps and other sampling methods. Distribution, habitat and substrate plant information is also noted on a standard recording form. The working group maintains a database of observations, literature and collection data of Coccinellidae from 1887 onwards. Preliminary atlases have been published (Branquart et al. 1999; Adriaens and Maes 2004) and updated distribution maps for the Belgian territory are available in Dutch and French online (http://www.inbo.be/docupload/2680.pdf).
The first feral field populations of H. axyridis in Belgium were discovered in 2001 (Adriaens et al. 2003). Since then, the Belgian Ladybird Working Group collected data on the spread and distribution of the species and also recorded data on its ecology in Belgium (Adriaens and Gysels 2002; San Martin et al. 2005). Collection events minimally consisted of species, number of individuals, stage (adults, larvae and pupae), observation date, observer and location. Locations were attributed to 1 × 1-km2 or 5 × 5-km2 grid cells of the UTM grid (Universal Transverse Mercator). At present, the database contains 53,458 records, of which 15% come from museum collections and literature data. A large part of the Belgian territory has now been surveyed for ladybirds: the database contains records for 85% of all 5 × 5-km UTM grid cells (N = 1,376) in Belgium. Additionally, information on substratum plants, height in the vegetation, sampling method, habitat type, surrounding landscape, slope orientation, soil type, humidity, vegetation cover and behaviour was noted on the recording form. Observers were also asked to record H. axyridis morph type following nomenclature based on literature (Komai 1956; Iablokoff-Khnzorian 1982; Serpa et al. 2003; Osawa and Nishida 1992): f. succinea, f. conspicua or f. spectabilis. The same nomenclature was applied in the UK’s Harlequin Ladybird Survey (Brown et al. 2007b) and The Netherlands’ Monitoring Project (Cuppen et al. 2004a, b). A small group of observers have performed detailed counts of morph type frequencies on different locations since the arrival of H. axyridis in Belgium in 2001.
Analysis
Distribution and invasion of H. axyridis in Belgium
To describe the distribution pattern, we used the Belgian ecoregions (Fig. 1) and calculated the number of 1-km² grid cells with and without presence of H. axyridis per ecoregion using 2001–2006 data. We used only well-prospected grid cells (i.e. with at least five native species recorded) and compared the presence/absence pattern in each ecoregion using χ2 tests.
We calculated the percentage of H. axyridis observations and the percentage of collection events expressed as the unique combination of [date × 1-km² grid cell × observer] with H. axyridis in the database per year (2001–2006). We only used collection events with more than one species observed to account for accidental observations. Geographic spread was analysed by calculating mean and maximal distances between centroids of grid cells with H. axyridis observations for the period 2001–2006 and taking 2001 as a starting point, using the find distance tool in ArcGis 3.2a Spatial Analist extension (ESRI). We used the Home Range Extension for ArcGIS (Rodgers and Carr 1998) to calculate the surface area of minimum convex polygons around 1-km² grid cell H. axyridis data (2001–2006) as a measure of invaded area.
Habitat preference
Absolute and relative number of H. axyridis observations (expressed as the unique combination of [date × 1-km² grid cell]) on different plant species and genera were calculated based on 1,349 data with substratum plant information. Based on post-2001 substratum plant species data, we also calculated the relative number of H. axyridis observations on different vegetation strata (trees, shrubs, dwarf-shrubs, herbs) and habitat types and compared that with patterns in native species using χ²-tests. For this analysis, we only included habitat types with at least 10 H. axyridis observations based on 2001–2006 data. Statistical analyses were performed using Statistica version 6.0 (StatSoft).
Potential interactions with native species
Three niche overlap like indices were calculated: two are based on the use of plant genera and one is based on spatio-temporal co-occurrence.
For each native ladybird species (38 species belonging to the subfamilies Coccinellinae, Chilocorinae, Epilachninae and Coccidulinae), we only used plants with at least two observations and we calculated plant indices only for ladybird species with at least 20 plant data (see Table 3).
We used Czekanowski index as an index of host plant use similarity (Feinsinger et al. 1981; Hurlbert 1978):
where p xi and p yi are the relative occurrences of species x and y on plant i. This index range from 0 (i.e. no resource shared) to 1 (i.e. all resources shared in the same proportion).
We also calculated Lloyd’s interspecific crowding index (Lloyd 1967):
where x i and y i are the absolute number of occurrences on plant i and X is the total number of occurrences of species x. This is a nonreciprocal index that gives a relative measure of the degree to which species y impinges on species x by the use of shared resources (Hurlbert 1978). This index can only be positive and a null value indicates no crowding at all. We calculated the degree to which H. axyridis impinges on native species and the degree to which native species impinge on H. axyridis.
For the spatio-temporal co-occurrence index, we used only collection events (expressed as the unique combination [date × 1-km² grid cell]) with at least two ladybird species observed. We considered only collecting events since 2004 when H. axyridis was already well established in Belgium. An estimate of probability of co-occurrence is given by the number of collecting events where the species x has been found with H. axyridis divided by total number of collecting events with species x.
Ecotope and landscape level analysis
We investigated the occurrence of H. axyridis in areas of conservation concern both on the ecotope and the landscape level. Therefore, we compared the average area of very valuable, valuable and less valuable land according to the Biological Valuation Map (BVM, version 2.0) for Flanders (north Belgium; Wils et al. 2006) in 1-km² grid cells with and without H. axyridis using a two-way ANOVA. We only used well-prospected grid cells i.e. cells in which at least five native species were recorded. The BVM is a uniform field-driven survey of the land cover and vegetation in north Belgium. This survey is translated into a biological valuation, largely based on plant species and vegetations. The biological value of legend units is fixed and determined by a number of ecological criteria: rarity of the biotope, presence or absence of certain species, biodiversity of the biotope, vulnerability and ‘replaceability’ of the biotope. Areas of very valuable, valuable and less valuable land were calculated using ArcMap (ESRI). The same analysis was performed for Belgium using CORINE Land Cover (Nunes de Lima 2005), using label 1 Land Cover classes (agricultural areas, artificial areas, forests and semi-natural areas, water bodies and wetlands) and label 3 Land Cover classes (beaches/dunes/sands, broad-leaved forest, complex cultivation patterns, coniferous forest, (dis)continuous urban fabric, estuaries, fruit trees and berry plantations, green urban areas, inland marshes, intertidal flats, land principally occupied by agriculture with significant areas of natural vegetation, mixed forest, moors and heathland, natural grasslands, pastures, peat bogs, road and rail networks and associated land, woodland-shrub, water bodies and water courses).
Phenology
The phenology of adults, larvae and pupae was investigated by calculating the number of observations (date × 1-km² grid cell) of H. axyridis (2,474 adult, 329 larval/pupal observations of H. axyridis) and other native species (Coccinellinae, Coccidulinae, Chilocorinae, Epilachninae) per 15-day period. Given the fact that recording efforts are fairly evenly spread over the different months of the investigated years, we do not expect a bias in phenology due to recording effort. Due to the limited number of data, observations of larvae and pupae were pooled. For native species, we used data from 1999 onward (22,790 adult, 918 larval/pupal observations), as this was the official starting date of the mapping project.
Morph types
We used a log-linear analysis of frequency tables to check for differences in two H. axyridis morph types (melanic = f. spectabilis and f. conspicua versus non-melanic = f. succinea) frequency among three consecutive years (2004–2006).
Results
Invasion and distribution in Belgium
Figure 2 shows the trend in the number of field observations of H. axyridis in Belgium until 2007. Although H. axyridis has been sold in Belgium by Koppert since 1996 (Poutsma et al. 2007) and was commonly used for biological control in Belgium since 1997 (B. Adam, pers. comm.), no observations in the wild were reported until September 2001 when the species was discovered in the cities of Ghent (28/09/2001) and Brussels (single adult on 22/10/2001). In Ghent, 27 adults (of which several freshly emerged individuals), three larvae and one pupa were discovered beating Acer platanoides L. and sweep netting Solidago canadensis L. vegetation in an urban park.
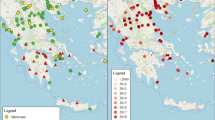
Since then, the number of H. axyridis observations increased (Fig. 2) and the species gradually expanded its range in Belgium (Fig. 3). The relative number of H. axyridis observations increased from 0.26% in 2001 to 23% in 2007 (Fig. 2). Also, in 2007, the relative number of ladybird collection events with H. axyridis has increased to 64%, compared to 7% in 2002 (Fig. 2).
The species rapidly spread to the east, the west and the south of Belgium (Fig. 3). In 2002, numerous feral populations were discovered in all but the westernmost Belgian provinces and in south Belgium. By the end of 2004, the species had colonised the whole Belgian territory with the exception of the southernmost parts of the country. By that time, H. axyridis had also started to spread in The Netherlands (Cuppen et al. 2004a, b), the UK (Brown et al. 2007a) and northern France (Coutanceau 2006).
In 2005 and 2006, the species was recorded in the southernmost parts of Belgium (Fig. 3). Initially, most observations in Belgium originated from cities and anthropogenic sites, and some could be attributed to escapes from biological control in nearby greenhouses. However, individuals were subsequently found in (semi-) natural habitats such as forests, wetlands, meadows and heathlands. Observations of eggs, larvae and pupae became numerous. Since the end of October 2002, relatively small overwintering aggregations (10–30 individuals) were observed in houses. The first large overwintering group was found in November 2002 in a concrete bunker of an old fortress in the surroundings of Antwerp. This aggregation consisted of several thousands of H. axyridis mixed with a few A. bipunctata individuals. Meanwhile, the phenomenon of October swarming and overwintering aggregates became quite common in Belgium. However, findings of very large overwintering groups, as reported in North America with estimates of hundreds of thousands individuals (Kidd et al. 1995), remained scarce. Only 17 observations were reported of overwintering aggregations of more than 500 individuals. Most aggregations were found inside private houses and concerned groups of 10–500 individuals.
Mean spreading distance increased over the period 2001–2006, which indicates that the species is still colonising new areas in Belgium (Fig. 4). The largest maximum colonisation distance was recorded between 2004 and 2005. Mean distances per year decreased, indicating a slow-down of the expansion (Fig. 4). The area of minimum convex polygons around occupied 1-km2 grid cells by H. axyridis increased from almost 10,000-km2 in 2002 to 31,000-km2 in 2006, and inclined towards the total area of Belgium (32,545-km2—Fig. 4). The number of grid cells occupied by H. axyridis was significantly higher in the sandy and loamy ecoregions Campine (χ²1 = 124.53, P < 0.001), Loam (χ²1 = 9.24, P = 0.024) and Sandyloam (χ²1 = 95.98, P < 0.001) and was lower in the four southernmost, less urbanised Belgian ecoregions: the hilly loess-soil ecoregion Condroz (χ²1 = 198.65, P < 0.001), the Fagne-Famenne-Calestienne (χ²1 = 85.68, P < 0.001), a region with a lot of chalk hills and calcareous grasslands, the Ardennes, the highest, wet and cold Belgian region with a lot of spruce stands and some moorlands (χ²1 = 124.53, P < 0.001) and the warm, calcareous southernmost ecoregion Gaume and Lorraine (χ²1 = 48.4, P < 0.001).
Habitat preference
A total of 1,688 H. axyridis observations (40%, 24,543 individuals) had associated plant use data. In total, H. axyridis was found on 159 plant species, belonging to 139 genera. The number of observations of adult H. axyridis was highest on nettle (Urtica dioica L.) and on deciduous trees such as maple (Acer sp.), willow (Salix sp.), lime (Tilia sp.), oak (Quercus sp.) and birch (Betula sp.) but the number of observations was also high on pine tree (Pinus sp.), hawthorn (Crataegus sp.) and on a number of herbs such as reed Phragmites australis Steud (Table 1). Fewer data were available for H. axyridis larvae and pupae compared to adults but preliminary analysis showed the aforementioned tree species to be the most important plants for larvae and pupae as well.
The relative occurrence of H. axyridis on different vegetation strata was comparable to that of native species (Table 2) indicating that H. axyridis occurrence on different vegetation strata is proportional to the sampling effort. More than half of the H. axyridis observations (52%) were done on trees, about one-third (34%) was done on herbs and 14% on shrubs. The use of dwarf shrubs (such as Calluna and Vaccinium) seems to be lower for H. axyridis (0.1%) than for other native species (2.4%).
Habitat data on H. axyridis were available for a total of 2,005 records. The species occurred in a variety of habitat types, both in anthropogenic habitats (parks and gardens, road verges, arable fields, pastures, orchards, fallow land, abandoned railways, forest clearings, coal mine spoil piles) as in more natural habitats with conservation value (forest, wood fringes, river banks/lake shores, brushwood, scrub/hedgerows, heathlands, swamps, meadows, marches, marshland, dunes, reed-lands, poor dry grasslands and calcareous grasslands). The relative number of H. axyridis observations was significantly higher than native species in parks and gardens (χ²1 = 22.44, P < 0.001), arable fields (χ²1 = 23.74, P < 0.001), orchards (χ²1 = 68.95, P < 0.001), brushwood (χ²1 = 6.55, P < 0.011) and reed-lands (χ²1 = 7.15, P < 0.008) and significantly lower in heathlands (χ²1 = 31.95, P < 0.001), pioneer vegetation (χ²1 = 8.96, P < 0.003), poor dry grasslands (χ²1 = 8.87, P < 0.003), dunes (χ²1 = 11.55, P < 0.001) and calcareous grasslands (χ²1 = 18.26, P < 0.001).
Potential interactions with native ladybirds
The plant use and co-occurrence indices are given in Table 3 along with the number of data used for the calculations and the number of plant genera with at least two observations for each ladybird species. The native ladybirds have been grouped according to the main biotopes used in Belgium (Branquart et al. 1999; Baugnée et al. 2001; Adriaens and Maes 2004).
The plant use similarity was highest with the four generalist species (45–77%) and particularly with A. bipunctata and Propylea quatuordecimpunctata (L.) (Coleoptera: Coccinellidae). The two other species, C. septempunctata and Psyllobora vigintiduopunctata (L.) (Coleoptera: Coccinellidae) are generalist species that are regularly found in all vegetation strata but show a clear preference for the herb layer. Most of the trees and deciduous trees ladybird species also showed quite high similarity values (around 50%). The plant use similarity was generally lower for the species living on coniferous trees, in heathlands, or in the herb layer of diverse biotopes.
The interspecific crowding was highly asymmetric: H. axyridis impinged potentially more on native species than the reverse, according to plant use. The only native species that showed higher interspecific crowding on H. axyridis were three generalist ladybirds: P. quatuordecimpunctata, A. bipunctata and C. septempunctata. The indices of interspecific crowding of H. axyridis on native species were highest for ladybirds living on coniferous trees and particularly for the three pine trees specialists: Myzia oblongoguttata (L.), H. quadripunctata and Myrrha octodecimguttata (L.) (Coleoptera: Coccinellidae). The potential interactions were also particularly high for generalist and deciduous trees ladybirds and for the rare myrmecophilous specialist Coccinella magnifica Redtenbacher (Coleoptera: Coccinellidae). The potential negative effects of H. axyridis on native ladybirds seemed to be lower on some species relatively to other native ladybirds: Exochomus nigromaculatus (Goeze) (a rare dry heathland specialist), Tytthaspis sedecimpunctata (L.) (a mycophagous herb layer species living mainly on grasses) and Epilachna argus (Geoffroy) (a phytophagous species living only on Bryonia dioica Jacq.) (Coleoptera: Coccinellidae).
The spatio-temporal co-occurrence with H. axyridis was very high for almost all species: most native ladybirds were found together with H. axyridis in 40–65% of the collecting events. There were no clear differences among the groups of species living in different biotopes. Only two species were found with H. axyridis in less than 30% of the observations: Coccinella hieroglyphica L. (a heathland specialist: 20% of the observations) (Coleoptera: Coccinellidae) and E. argus (27%).
Land cover analysis
Grid cells with H. axyridis had more area of less valuable biotopes and less area of very valuable biotopes according to the BVM than grid cells where the species was not observed (F (2, 1887) = 10.901, P < 0.001—Fig. 5). The area of agricultural area (F 1 = 6.485, P = 0.011), artificial surfaces (F 1 = 3.857, P = 0.050), forest and semi-natural areas (F 1 = 7.391, P = 0.007) was significantly larger in grid cells with H. axyridis than in grid cells without the species. Using label 3 CORINE land cover classes, grid cells with H. axyridis had a significantly larger area of urban fabric (F 1 = 6.625, P = 0.01), estuaries (F 1 = 4.700, P = 0.030) and land principally occupied by agriculture with significant areas of natural vegetation (F 1 = 9.122, P = 0.003). Grid cells with H. axyridis had a significantly smaller area of broad-leaved forest (F 1 = 10.04, P = 0.002), mixed forest (F 1 = 21.403, P < 0.001), moors and heathland (F 1 = 6.881, P 1 = 0.009), peat bogs (F 1 = 6.528, P = 0.011) and water courses (F 1 = 4.468, P = 0.035).
Phenology
Our field data showed that H. axyridis adults were found throughout the summer, from the second half of April–first half of May to September, with a peak in the first half of August and two smaller peaks in June and September. A second peak for H. axyridis adults appeared in the second half of October, about 2 months later than native species and was probably related to swarming behaviour. A peak of immature stages of H. axyridis appeared in between the two summer peaks of adults, around the second half of June, and again in the first half of October. By May–June 2007 there were indeed several records of large groups of H. axyridis larvae crawling onto sunny garden walls, walls of houses, churches and cars to pupate, causing (aesthetic) nuisance to people. Adults of native species showed only two peaks, the first half of May and the first half of August, with a single peak of immature stages in between around the first half of July (Fig. 6).
Morph types
A total of 748 records (out of 5,164) of H. axyridis had information on morph type. The form succinea was the most commonly encountered morph type (72%), followed by spectabilis (19%) and conspicua (5%). About 4% belonged to one of two melanic morphs conspicua/spectabilis. These relative frequencies were comparable to the frequencies in counted overwintering groups (N = 1,838): 70% succinea, 18% spectabilis, 8% conspicua and 5% conspicua/spectabilis and to the frequencies in various single populations. The ratio of melanic and non-melanic H. axyridis did not differ among years (2004–2006) (Log linear analysis χ²3 = 0.488, P = 0.92).
Discussion
About 4 years after its discovery in 2001 in the north of Belgium, H. axyridis had already spread throughout the Belgian territory. Based on its current distribution in Belgium, H. axyridis can clearly be considered as a eurytopic species with large amplitude for geomorphologic and climatic conditions in Belgium. Analysis of occupied and well-prospected unoccupied grid cells however, showed that the species is less common in the southern parts of Belgium. This pattern might be influenced by lower sampling effort in the southern part of Belgium. However, we believe it is the result of invasion history of the species since the species is less common on typical ecotopes present in these regions e.g. calcareous grasslands, moorlands and forests. The species showed a clear preference for the sandy and loamy regions in the north of Belgium. These are also the regions that are most species-rich in native coccinellids (Adriaens and Maes 2004). Hotspots for other arthropod groups, such as butterflies, are also located here (Maes et al. 2005).
The results on substratum plants were only partly consistent with literature. H. axyridis is generally regarded a (semi-)arboreal species, occurring mostly on deciduous trees (e.g. Hodek 1973; Iablokoff-Khnzorian 1982) but also in various herbaceous habitats (Koch et al. 2006). In Belgium, H. axyridis was indeed observed mostly on deciduous trees, but was also frequently found on pine trees and in the herb layer. Iablokoff-Khnzorian (1982) mentioned H. axyridis on pine trees in China, but added that this might in fact concern H. yedoensis (Takizawa) (Coleoptera: Coccinellidae). Our results clearly showed that, at least in Belgium, H. axyridis frequently uses pine trees. However, these results have to be interpreted with care. As aphids are more scattered in the herb layer an effect of dilution might occur that could explain the lower number of individuals on herbs. Moreover, sweeping, generally, does not allow the observer to identify individual plant species in the herb layer and observers were probably more able to determine tree species than herb species.
Plant use and co-occurrence indices only give a coarse idea of the potential impact of H. axyridis on native species because they do not take into account the real trophic resources (aphids or other prey that are not necessarily limiting) and intra-guild predation events (Wissinger 1992). But co-occurrence and shared habitat are preliminary conditions for competition and intra-guild predation (Wissinger 1992). Based on the niche overlap analyses, generalist and deciduous tree ladybirds are probably the most negatively affected native species. Moreover, our results showed that pine tree specialist ladybirds, although they have a quite different niche, could also potentially be affected by H. axyridis interactions because they are highly specialised on a resource frequently used by H. axyridis. It seems that the most threatened and localised species, heathland and wetland specialists, would be less affected relative to other species. This is consistent with the results on habitat preference and the area of different land cover classes according to the biological valuation and CORINE land cover maps. Few methods are presently available to control H. axyridis in natural environments (Kenis et al. 2007). Our data suggest that control measures should focus on managing H. axyridis populations outside semi-natural areas (e.g. mechanical methods to control aggregation) to prevent the species spreading into more natural areas of conservation concern.
Habitat data showed that H. axyridis is frequently found in a broad range of semi-natural biotopes. However, biotope and landscape level data and data on land cover showed that the species is more frequently found in more urbanised and anthropogenic landscapes and less frequent in landscapes with forests and other natural elements (moors and heathland, peat bogs, water courses). This could explain the apparent slower colonisation rate of the four southernmost natural ecoregions that are less urbanised and much more wooded.
Harmonia axyridis is considered bivoltine in most of its range. In central Japan, the species had a bivoltine cycle (Sakurai et al. 1988). In its invasive range, H. axyridis was also reported as bivoltine, e.g. in a Minnesota agricultural landscape (Koch and Hutchison 2003) and in Oregon (Lamana and Miller 1996). However, up to four or five generations per year have been observed, for example in Italy (Bazzocchi et al. 2004) and Greece (Katsoyannos et al. 1997). In southern France, two generations were reported (Ongagna et al. 1993) and evidence was presented of bi-voltinism in the UK population (Brown et al. 2007b). The same seems to be true for the Belgian H. axyridis population. Despite species-specific variations, the main pupal eclosion period for most native ladybird species is from mid-July to mid-August (Majerus 1994). The adult phenology of native species, based on the number of observations, is consistent with this pattern, clearly showing two adult peaks in May and August and a July peak of immature stages in between. Harmonia axyridis adults and immature stages, however, peak again in October, very late in the season and about 2 months later than native species. This might indicate that H. axyridis is more able to exploit alternative resources and reproduce in the absence of aphid prey. Berkvens et al. (2007) also suggested that H. axyridis might have a competitive advantage over indigenous species because it can exploit pollen when insect prey is scarce. Immature stages of first generation H. axyridis showed an overlap with native species, indicating a high potential for intraguild predation (Pell et al. 2007).
The extreme phenotypic variability in colour morphs of H. axyridis appears to have a genetic basis (for a review see Komai 1956). The occurrence of different colour morphs is subject to spatial variation (Dobzhansky 1933) and could give information on the origin of H. axyridis in Belgium. The observed frequencies of the various colour morphs in Belgium were largely the same as in the rest of Europe, with a mixture of the three morphs present and with f. succinea predominating (Brown et al. 2007a; Majerus and Roy 2005). This might provide evidence that introduced H. axyridis in Belgium came from a population with a homogenous colour morph profile. We could find no evidence of a decline in the percentage of melanic forms, as has been reported in the UK where it has been related to a separate introduction of a population with a different colour morph profile (Brown et al. 2007a). The equicolor morph type, observed several times in The Netherlands, has not yet been recorded in Belgium.
We conclude that large-scale field data provide valuable information on the invasion process and the potential negative impact of H. axyridis on native ladybird species. Future work on feral H. axyridis populations in Belgium is needed to estimate its impact on the indigenous fauna, aphidophagous or other, and to acquire insights in the underlying mechanisms. Until now, monitoring the abundance of H. axyridis and native ladybirds has been restricted to the urban environment of Brussels in Belgium. More quantitative data are, however, needed to determine the exact impact of H. axyridis on the native fauna in the invasion range. A more extensive monitoring scheme comprising different habitat types, including those with a high conservation value, should be applied to assess the impact of this invasive alien.
References
Adriaens T, Gysels J (2002) Veelkeurig aziatisch lieveheersbeestje, van biologische bestrijder tot pestsoort? Natuur Focus 1(4):148–152
Adriaens T, Maes D (2004) Voorlopige verspreidingsatlas van lieveheersbeestjes in vlaanderen, resultaten van het lieveheersbeestjesproject van de jeugdbonden. Bertram 2(1bis):1–69
Adriaens T, Branquart E, Maes D (2003) The multicoloured Asian ladybird Harmonia axyridis Pallas (Coleoptera : Coccinellidae), a threat for native aphid predators in Belgium? Belg J Zool 133(2):195–196
Alyokhin A, Sewell G (2004) Changes in a lady beetle community following the establishment of three alien species. Biol Invasions 6:163–471
Baugnée J-Y, Branquart E, Maes D (2001) Velddeterminatietabel voor de lieveheersbeestjes van België (Chilocorinae, Coccinellinae en Epilachninae). Jeugdbond voor Natuurstudie en Milieubescherming (Gent), Jeunes & Nature asbl (Wavre) i.s.m. Instituut voor Natuurbehoud (Brussel)
Bazzocchi GG, Lanzoni A, Accinelli G, Burgio G (2004) Overwintering, phenology and fecundity of Harmonia axyridis in comparison with native coccinellid species in Italy. BioControl 49(3):245–260
Berkvens N, Bonte J, Berkvens D, Deforce K, Tirry L, De Clercq P (2007) Pollen as an alternative food for Harmonia axyridis. BioControl (this issue). doi:10.1007/s10526-007-9128-7
Branquart E, Baugnée J-Y, Mairesse JL, Gaspar C (1999) Inventaire de la faune des coccinelles de Wallonie (Chilocorinae, Coccinellinae et Epilachninae). Rapport final. Gembloux
Brown MW, Miller SS (1998) Coccinellidae (Coleoptera) in apple orchards of Eastern West Virginia and the impact of invasion by Harmonia axyridis. Entomological News 109(2):143–151
Brown PMJ, Adriaens T, Bathon H, Cuppen J, Goldarazena A, Hägg T, Kenis M, Klausnitzer BEM, Kovář I, Loomans AJM, Majerus MEN, Nedved O, Pedersen J, Rabitsch W, Roy HE, Ternois V, Zakharov IA, Roy DB (2007a) Harmonia axyridis in Europe: spread and distribution of a non-native coccinellid. BioControl (this issue). doi:10.1007/s10526-007-9132-y
Brown PMJ, Roy HE, Rothery P, Roy DB, Ware RL, Majerus MEN (2007b) Harmonia axyridis in Great Britain: analysis of the spread and distribution of a non-native coccinellid. BioControl (this issue). doi:10.1007/s10526-007-9124-y
Burgio G, Santi F, Maini S (2002) On intra-guild predation and cannibalism in Harmonia axyridis (Pallas) and Adalia bipunctata L. (Coleoptera: Coccinellidae). Biol Control 24(2):110–116
Colunga-Garcia M, Gage SH (1998) Arrival, establishment, and habitat use of the multicolored Asian lady beetle (Coleoptera: Coccinellidae) in a Michigan landscape. Environ Entomol 27(6):1574–1580
Cottrell TE, Yeargan KV (1998) Intraguild predation between an introduced lady beetle, Harmonia axyridis (Coleoptera : Coccinellidae), and a native lady beetle, Coleomegilla Maculata (Coleoptera: Coccinellidae). J Kans Entomol Soc 71(2):159–163
Coutanceau J (2006) Harmonia axyridis (Pallas, 1773): une coccinelle asiatique introduite, acclimatée et en extension en France. Bull Soc entomol Fr 111(3):395–401
Cuppen J, Heijerman Th, van Wielinck P, Loomans AJM (2004a) Het lieveheersbeestje Harmonia axyridis in Nederland: een aanwinst voor onze fauna of een ongewenste indringer (Coleoptera: Coccinellidae)? Nederlandse Faunistische Mededelingen 20:1–12
Cuppen J, Heijerman Th, van Wielinck P, Loomans AJM (2004b) Veelkleurig aziatisch lieveheersbeestje in opmars. Natura 66(3):3
De Clercq P, Bonte M, Van Speybroeck K, Bolckmans K, Deforce K (2005) Development and reproduction of Adalia bipunctata (Coleoptera: Coccinellidae) on eggs of Ephestia kuehniella (Lepidoptera: Phycitidae) and pollen. Pest Manag Sci 61(11):1129–1132
Dobzhansky T (1933) Geographical variation in lady beetles. Am Nat 67:97–126
Feinsinger P, Spears E, Poole RW (1981) A simple measure of niche breadth. Ecology 62(1):27–32
Ferran A, Niknam H, Kabiri F, Picart JL, Deherce C, Brun J, Iperti G, Lapchin L (1996) The use of Harmonia axyridis larvae (Coleoptera: Coccinellidae) against Macrosiphum Rosae (Hemiptera: Sternorrhyncha: Aphididae) on rose bushes. Eur J Entomol 93(1):59–67
Gordon RD (1985) The Coleoptera (Coccinellidae) of America north of Mexico. J New York Entomol Soc (93):1–912
Hautier L (2003a) Impacts sur l’entomofaune indigène d’une coccinelle exotique utilisée en lutte biologique. TFE Université Libre de Bruxelles, IGEAT, Brussels
Hautier L (2003b) Intraguild predation of Adalia bipunctata by Harmonia axyridis. Presentation. Workshop Ladybirds and biological control in Belgium, Brussels, November 2003
Hautier L (2006) Intraguild predation by Harmonia axyridis. In: Branquart E, Baus E, Pieret N, Vanderhoeven S, Desmet P (eds) SOS Invasions—uitheemse invasieve soorten in België, Brussels, March 9, 2006
Hironori Y, Katsuhiro S (1997) Cannibalism and interspecific predation in two predatory ladybirds in relation to prey abundance in the field. Entomophaga 42(1–2):153–163
Hodek I (1973) Life history and biological properties. In: Hodek I (ed) Biology of Coccinellidae. Dr. W. Junk, The Hague
Hurlbert SH (1978) The measurement of niche overlap and some relatives. Ecology 59(1):67–77
Iablokoff-Khnzorian SM (1982) Les coccinelles (Coleoptera: Coccinellidae). Tribu Coccinellini des régions Paléarctique et Orientale. Boubée, Paris
Iperti G, Bertrand E (2001) Hibernation of Harmonia axyridis (Coleoptera: Coccinellidae) in South-Eastern France. Acta Soc Zool Bohem (65):207–210
Katsoyannos P, Kontodimas DC, Stathas GJ et al (1997) Establishment of Harmonia Axyridis on citrus and some data on its phenology in Greece. Phytoparasitica 25(3):183–191
Kenis M, Roy HE, Zindel R, Majerus MEN (2007) Current and potential management strategies against Harmonia axyridis. BioControl (this issue). doi:10.1007/s10526-007-9136-7
Kidd KA, Nalepa CA, Day ER, Waldvogel MG (1995) Distribution of Harmonia Axyridis (Pallas) (Coleoptera, Coccinellidae) in North-Carolina and Virginia. Proc Entomol Soc Wash 97(3):729–731
Koch RL (2003) The multicolored Asian lady beetle, Harmonia axyridis: a review of its biology, uses in biological control, and non-target impacts. J Insect Sci 3(32):1–16
Koch RL, Hutchison WD (2003) Phenology and blacklight trapping of the multicolored Asian lady beetle (Coleoptera: Coccinellidae) in a Minnesota agricultural landscape. J Entomol Sci 38(3):477–480
Koch RL, Venette RC, Hutchison WD (2006) Invasions by Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) in the Western Hemisphere: implications for South America. Neotrop Entomol 35(4):421–434
Komai T (1956) Genetics of ladybeetles. Adv Genet 8:155–188
Lamana ML, Miller JC (1996) Field observations on Harmonia axyridis Pallas (Coleoptera: Coccinellidae) in Oregon. Biol Control 6(2):232–237
Lloyd M (1967) ‘Mean crowding’. J Anim Ecol 36(1):1–30
Maes D, Bauwens D, De Bruyn L, Anselin A, Vermeersch G, Van Landuyt W, De Knijf G, Gilbert M (2005) Species richness coincidence: conservation strategies based on predictive modelling. Biodivers Conserv 14(6):1345–1364
Maignet P (2002) Bilan de l’introduction en France de la coccinelle Harmonia axyridis Pallas en lutte biologique contre les pucerons. Deuxième Conférence Internationale sur les Moyens Alternatifs de Lutte contre les Organismes Nuisibles aux Végétaux, March 4, 2002
Majerus MEN (1994) Lady birds. Harper-Collins, London
Majerus MEN, Roy DB (2005) Scientific opportunities presented by the arrival of the Harlequin ladybird, Harmonia axyridis, in Britain. Antenna 29:196–208
Michaud JP (2002) Invasion of the Florida citrus ecosystem by Harmonia axyridis (Coleoptera: Coccinellidae) and asymmetric competition with a native species, Cycloneda Sanguinea. Environ Entomol 31(5):827–835
Michaud JP (2004) Natural mortality of Asian citrus psyllid (Homoptera: Psyllidae) in central Florida. Biol Control 29(2):260–269
Nunes de Lima MV (2005) CORINE land cover (updating for the year 2000). European Commission, Italy
Ongagna P, Giuge L, Iperti G, Ferran A (1993) Life-cycle of Harmonia axyridis (Col, Coccinellidae) in its area of introduction—South-Eastern France. Entomophaga 38(1):125–128
Osawa N (2000) Population field studies on the Aphidophagous ladybird beetle Harmonia axyridis (Coleoptera: Coccinellidae): resource tracking and population characteristics. Popul Ecol 42(2):115–127
Osawa N, Nishida T (1992) Seasonal-variation in elytral color polymorphism in Harmonia axyridis (the ladybird beetle)—the role of nonrandom mating. Heredity 69:297–307
Ottart N (2005) Impacts de la coccinelle invasive Harmonia axyridis sur les populations de coccinelles indigènes à Bruxelles. TFE Université Libre de Bruxelles, Ecole Interfacultaire de BioIngénieur, Brussels
Pell JK, Baverstock J, Roy HE, Ware RL, Majerus MEN (2007) Intraguild predation involving Harmonia axyridis: a review of current knowledge and future perspectives. BioControl (this issue). doi:10.1007/s10526-007-9125-x
Poutsma J, Loomans AJM, Aukema B, Heijerman T (2007) Predicting the potential geographical distribution of the harlequin ladybird, Harmonia axyridis using the CLIMEX model. BioControl (this issue). doi:10.1007/s10526-007-9140-y
Rodgers AR, Carr AP (1998) HRE: The Home Range Extension for ArcView. Thunder Bay, Ontario, Canada, Ontario Ministry of Natural Resources, Centre for Northern Forest Ecosystem Research
Sakurai H, Takeda S, Kawai T (1988) Diapause regulation in the lady beetle, Harmonia axyridis. In: Niemczyk E, Dixon AFG (eds) Ecology and effectiveness of Aphidophaga, S.P.B. Academic Publishing, The Hague
San Martin G. (2003) Etude de l’impact de l’urbanisation sur les populations de coccinelles à Bruxelles. TFE Université Libre de Bruxelles, Faculté des Sciences, Service d’Eco-Ethologie Evolutive
San Martin G, Adriaens T, Hautier L, Ottart N (2005) La coccinelle asiatique Harmonia axyridis. Insectes 136(1):7–11
Serpa L, Schanderl H, Brito C, Soares AO (2003) Temporal and geographic variations in elytral colour of the polymorphic ladybird beetle Harmonia axyridis Pallas (Coleoptera:Coccinellidae): the role of fitness and genetic transmission. In: Proceedings of the 8th international symposium on ecology of Aphidophaga
Tedders WL, Schaefer PW (1994) Release and establishment of Harmonia axyridis (Coleoptera, Coccinellidae) in the Southeastern United States. Entomol News 105(4):228–243
van Lenteren JC, Babendreier D, Bigler F, Burgio G, Hokkanen HTM, Kuske S, Loomans AJM, Menzler-Hokkanen I, van Rijn PCJ, Thomas MB, Tommasini MG, Zeng Q-Q (2003) Environmental risk assessment of exotic natural enemies used in inundative biological control. BioControl 48(1):3–38
van Lenteren JC, Loomans AJM, Babendreier D, Bigler F (2007) Harmonia axyridis: an environmental risk assessment for Northwest Europe. BioControl (this issue). doi:10.1007/s10526-007-9120-2
Williamson M (2006) Explaining and predicting the success of invading species at different stages of invasion. Biol Invasions 8(7):1561–1568
Wils C, Paelinckx D, Adams Y, Berten B, Bosch H, De Knijf G, De Saeger S, Demolder H, Duelinckx R, Lust P, Oosterlynck P, Scheldeman K, t'Jollyn F, Van Hove M, Vandebussche V, Vriens L (2006) Biologische Waarderingskaart van het Vlaamse Gewest. Instituut voor Natuur- en Bosonderzoek (INBO), Brussels
Wissinger SA (1992) Niche overlap and the potential for competition and intraguild predation between size-structured populations. Ecology 73:1431–1444
Yasuda H, Ohnuma N (1999) Effect of cannibalism and predation on the larval performance of two ladybird beetles. Entomol Exp Appl 93(1):63–67
Yasuda H, Kikuchi T, Kindlmann P, Sato S (2001) Relationships between attack and escape rates, cannibalism, and intraguild predation in larvae of two predatory ladybirds. J Insect Behavior 14(3):373–384
Acknowledgements
We are very grateful to the numerous volunteers of the Belgian Ladybird Working Group for providing us with the field data on H. axyridis in Belgium. We thank three anonymous referees for useful comments on the manuscript. We are grateful to Helen Roy for correcting the English and to Dirk Bauwens for help in statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adriaens, T., San Martin y Gomez, G. & Maes, D. Invasion history, habitat preferences and phenology of the invasive ladybird Harmonia axyridis in Belgium. BioControl 53, 69–88 (2008). https://doi.org/10.1007/s10526-007-9137-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-007-9137-6