Abstract
Verticillium wilt is an important disease in oilseed rape with an increasing importance worldwide. Currently, there are no methods available to suppress the pathogen. A biological protection strategy on the basis of the plant-beneficial bacterium Serratia plymuthica HRO-C48 to control Verticillium dahliae in oilseed rape was developed. Three different techniques to apply the biocontrol agent to seeds, namely pelleting, film coating and bio-priming, were evaluated considering the influence on the control activity, cell stability during storage and practical feasibility. Neither the treatment nor the inoculum density was found to influence the abundances of HRO-C48 in the rhizosphere after 30 days. Serratia treatment using bio-priming and pelleting resulted in a statistically significant biocontrol in comparison to the non-bacterized controls. Additionally, survival of HRO-C48 differed between treatments, and was the highest using bio-priming at 20°C, and pelleting at 4°C. In conclusion, the procedure of bio-priming, which was developed in line with this study, resulted in a stable and efficient formulation of S. plymuthica on rape seed. This technology opens a possibility to develop a commercial Serratia formulation to protect oilseed against V. dahliae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Verticillium wilt caused by the soilborne fungus Verticillium dahliae is an important disease responsible for dramatic yield losses in many crops worldwide (Tjamos et al. 2000). Microsclerotia of Verticillium that develop in the senescing tissues of infested plants may persist in soil for several years in the absence of a susceptible host. Consequently, chemical control is nearly impossible. With the impending phase-out of the fumigant methyl bromide and related substances worldwide, there is a growing need for alternative management strategies (Martin et al. 2003). In addition, increasing practice of reduced crop rotations highly promote the accumulation of microsclerotia in agricultural soils. However, some soil amendments, e.g. broccoli residues, lignin or liquid swine manure, decrease the infection potential of Verticillium (Subbarao et al. 1999; Conn and Lazarovits 2000; Debode et al. 2002). On the other hand, the broad host range reduce the potential of crop rotation in suppressing the pathogen. Therefore, new solutions to protect Verticillium host plants against the pathogen are urgently needed.
In the last 20 years, an increase of 260% in oilseed production has been recorded worldwide which resulted in a yield of 36 million tons in 2003 (FAO, Statistical Databases). The crop belongs to renewable material and is one of the most important oil crops worldwide. However, Verticillium wilt has become a serious problem in oilseed rape, and, currently, no possibility to suppress the pathogen is available. The identity and molecular biology of the wilting pathogen in oilseed rape was unclear for a long time. Stark (1961) was the first person to report a Verticillium isolate with long spores, which he called V. dahliae var. longisporum. This group of Verticillium “longisporum” has a specific host range, which preferential include oilseed rape and horseradish, and is characterized as a different phenotype (Zeise and Tiedemann 2001). In 1997, V. longisporum was described as a new species (Karapapa et al. 1997). For both Verticillium species a very close relationship exists between the ITS sequences as well as the whole genome (Fahleson et al. 2004). In addition, both are able to induce wilting symptoms in oilseed rape although long-spored isolates of Verticillium were found to be the main pathogen (Steventon et al. 2002). As known today, these isolates are amphihaploid interspecific hybrids between V. dahliae and an unknown other Verticillium species (Barbara and Clewes 2003). In our study we have been working with strains which were originally isolated from oilseed rape and which belong to the long-spored form (Messner et al. 1996).
Biological control of soil-borne pathogens by naturally occurring antagonistic microorganisms may be one important step to environmentally friendly agriculture (Weller 1988; Emmert and Handelsman 1999; Whipps 2001). Previous studies have shown that antagonistic bacteria can be successfully used to suppress V. dahliae ad planta (Berg et al. 2001; Graner et al. 2003; Tjamos et al. 2004; Mercado-Blanco et al. 2004). For example, Serratia plymuthica HRO-C48, a rhizosphere-associated bacterium originally isolated from oilseed rape, was applied to protect strawberry roots against V. dahliae (Berg et al. 1999; Kurze et al. 2001a). The strain was selected as biocontrol agent according to the following criteria: (i) high antagonistic activity against several fungal pathogens in vitro (Kalbe et al. 1996; Frankowski et al. 2001); (ii) production of the plant growth hormone indole-3-acetic acid (Kalbe et al. 1996); (iii) harmless to human health and the environment; (iv) low level of antibiotic resistance (Berg 2000), and mainly (v) because of its biocontrol and plant growth promotion effect under field conditions (Kurze et al. 2001a). S. plymuthica cells could be reisolated from the rhizosphere until 14 month after the inoculation of strawberry roots, and the total bacterial community was not adversely affected (Kurze et al. 2001b; Scherwinski et al. 2007). A product on the basis of S. plymuthica HRO-C48 was developed and named RhizoStar® (e-nema GmbH, Raisdorf, Germany).
The objective of our research was to evaluate the effect of S. plymuthica in the pathosystem Verticillium—oilseed rape. Initially, an efficient formulation or seed treatment technique had to be developed which allow our biological control agent (BCA) to successfully establish Serratia in the rape rhizosphere. In contrast to the most spore forming, Gram positive bacteria, the formulation of Gram negative bacteria is more challenging, and one of the main reasons why numerous potential BCAs are not developed to commercially available products (Whipps et al. 1997; Whipps 2001). For our study, seeds of oilseed rape cv. Talent were treated with Serratia cells using pelleting, film coating, and bio-priming. These treatments were assessed by monitoring the establishment of Serratia in the rhizosphere, disease suppression, and plant growth promotion under optimal greenhouse conditions. Furthermore, the stability of the formulation was analyzed at different temperatures. In conclusion, using bio-priming to apply S. plymuthica to oilseed rape seeds resulted in promising findings.
Materials and methods
Microorganisms and culture conditions
Two derivatives of Serratia plymuthica strain HRO-C48 were used in the experiments. The wild type strain of S. plymuthica, which is deposited in the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany) under the accession number DSMZ 12502, was applied to seeds destined for the evaluation of biocontrol activity against V. longisporum. For colonization experiments a spontaneous mutant of S. plymuthica HRO-C48 resistant to 150 μg ml−1 rifampicin (Sifin, Berlin, Germany) were selected and designated as HRO-C48 Rif r. Isolates were cultured in nutrient broth in an orbital incubator for 24 h at 30°C. Microsclerotia of V. longisporum strain ELV 25 (Messner et al. 1996) were produced in Czapec Dox–vermiculite medium: 4 l of vermiculite (Vermiculite Dämmstoffe, Spröckhövel, Germany) were amended with 200 ml Czapek-Dox broth (Difco Laboratories, Detroit, USA). The mixture was inoculated with a 2-week old culture of V. longisporum (Czapek-Dox broth, 20°C at 120 rpm) and incubated at room temperature for 4 weeks.
Seed treatments
After cultivation of S. plymuthica bacterial cells were separated by centrifugation at 7,500 g and resupended in a medium according to the application method described in Fig. 1. All suspensions were adjusted to log10 10.0 CFU ml−1. Using pelleting 500 μl of cells suspended in 1.5% (w/v) methyl cellulose solution (Roth, Karlsruhe, Germany) were added to 2.0 g of seeds (cv. Talent, NPZ, Hohenlieth, Germany), which were placed in a lab-scaled pelleting machine. To bind remaining liquid 2.0 g talcum was added. Seeds were kept rotating in the machine until the seed coating was uniform. Using film coating 0.4 g of seeds was wetted in 1.0 ml of cell suspension containing 1.0% (w/v) sucrose. Afterwards, inoculated seeds were lyophilized for 5 h. For bio-priming 0.4 g of seeds were incubated for 12 h at 20°C in 2.0 ml of cells suspension containing 0.85% (w/v) sodium chloride. Seeds were agitated during incubation. Later, infiltrated seeds were dryed for 24 h at 20°C to the desired moisture content of about 5.0%. Treated seeds were stored at temperatures 4°C and 20°C, respectively, to observe the stability of S. plymuthica cells over a period of 30 days.
To determine the cell numbers per seed immediately after treatment and after storage, the average of three replicates in each treatment consisting of 20 seeds was calculated. The seeds were transferred into sterile 1.0 ml 0.85% (w/v) sodium chloride solution. Pelleted and film coated seeds were vortexed until the coatings of the seeds were removed completely. Primed seeds were grounded using an autoclaved motar and pestle. Suspensions were serially diluted with sterile 0.85% NaCl and plated onto NA medium in two replicated per dilution. Plates were incubated for 24 h at 30°C and colony forming units (CFU) were counted to calculate the means of colonies (log10 CFU).
Greenhouse experiments
The evaluation of the biocontrol activity against Verticillium wilt of differently formulated bacteria was carried out under greenhouse conditions. For the experiment, seeds were initially inoculated with a cell density of log10 6.0 seed−1. About 15 seeds in each treatment were sown in pots (one per pot) with a volume of 250 ml. Pots contained propagation compost (Einheitserdewerk, Uetersen, Germany) mixed with vermiculite and fungal inoculum (20:5:1, v/v/v). Plants were grown over a period of 9 weeks in a greenhouse at a temperature of 25 ± 10°C and assimilation light (16/8 h light/dark). They were watered every second day. After the appearance of first symptoms the disease reaction of plants was assessed by determining the severity of symptoms on a 1–9 scale (1 = no symptoms, 2 = few dark colored wires, 3 = oldest leave with strong symptoms, 4 = loss of the oldest leave, 5 = about 50% of leaves with strong symptoms, 6 = loss of about 50% of the leaves, 7 = loss of over 50% of the leaves, 8 = only the vegetation conus left, 9 = dead plant) at a weekly interval. Data on disease severity was used to calculate area under disease process curve (AUDPC) determined as \( {\text{AUDPC = }}{\sum {(({\text{S}}} }_{{\text{i}}} + {\text{S}}_{{{\text{ti+1}} }} )/2)^{\ast}({\text{ti}} + 1 - {\text{ti}}), \) where Si is the symptoms severity, and ti is the date of assessment of symptoms severity (Zeise 1992). To exclude the impact of the treatments by itself, a control experiment was carried out using seeds treated according to the protocol mentioned above (Fig. 1). The bacterial suspensions were substituted by a sterile 0.85% NaCl solution.
For the determination of colonization rates of Serratia plymuthica the initial cell density was adjusted to log10 6.0 CFU seed−1. Treated seeds (24 replicates in each treatment) were sown in pots (two per pot) containing propagation compost mixed with vermiculite (4:1 v/v). Plants were grown over a period of 60 days in greenhouse at a temperature of 25 ± 10°C and assimilation light (16/8 h light/dark). They were watered every second day. Roots with adhering soil from six plants in each treatment and sampling point were analyzed after 15, 30, 45 and 60 days. Finally, three samples per treatment each consisting of root material from two plants to a total weight of 5.0 g were obtained at each sampling time. To extract the rhizosphere microorganisms from the roots, the samples were placed into sterile bags, were amended with 50 ml sterile demineralized water and homogenized in a Stomacher laboratory blender for 180 s (BagMixer, Interscience, St. Nom, France). Soil suspensions were serially diluted in sterile 0.85% (w/v) NaCl and plated onto NA medium containing 100 μg ml−1 rifampicin in two duplicates per treatment and replicate. Plates were incubated for 24 h at 30°C and CFUs were counted to calculate the means of colonies (log10 CFU) based on the fresh weight.
Optimization of the bio-priming procedure
To specify the time course of germination of oilseed rape seeds used in this study, 1.0 g of seeds were covered by 5.0 ml 0.85% (w/v) NaCl solution. The increase of water content of the seeds over the time was determined by Moisture Analyzer MB 35 (Roth, Karlsruhe, Germany). After decanting remaining sodium chloride solution, adherent liquid was removed using filter paper. Water content of seeds was calculated after drying for 30 min at 125°C. The development of bacterial cell numbers inside the seed during bio-priming in correlation to the time course of germination were studied by treating seeds as described above. Samples were taken after 4, 12, and 20 h. To detect S. plymuthica cells inside the seed exclusively, before grounding, the seed surface was disinfested in NaClO (2.5% (v/v) available chlorine) for 5 min, and rinsed with demineralized water three times for 1 min.
Statistical analysis
Data on percentages of disease incidence and yield were analyzed for significance using Fisher’s protected least significance difference (P ≤ 0.05) by Statistical Product and Service Solutions for Windows, Rel. 9. 0. 1. (SSPS Inc., Chicago, IL, USA). Root colonization data were log10 transformed before statistical analysis.
Results
Establishment of S plymuthica HRO-C48 in the rhizosphere of oilseed rape
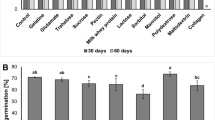
In a first greenhouse trial, the influence of the inoculum density of S. plymuthica HRO-C48 applied to seeds of oilseed rape on the establishment of the BCA in the rhizosphere was investigated. For this purpose, the technique of film coating was used because this treatment resulted in most accurate CFU per seed. Initial bacterial cell numbers from log10 3.0 to 7.0 CFU seed−1 of the spontaneous rifampicin resistant mutant of S. plymuthica HRO-C48 Rif r were applied. Data in Fig. 2A demonstrate that no statistically significant differences P ≤ 0.05 in plate counts of re-isolated bacteria were found. After 30 days, abundances of log10 4.7 ± 0.04 CFU g−1 root fresh mass (rfm) on average were found.
(A) Influence of different initial cell concentrations on the establishment of S. plymuthica HRO-C48 in the rhizosphere. Oilseed rape seeds were treated with S. plymuthica HRO-C48Rif r (spontaneous rifampicin resistant mutants) cells using film coating at concentrations of log10 3 to 7 CFU seed−1. After 30 days of growth under controlled greenhouse conditions bacteria were re-isolated on rifampicin-containing agar (nutrient agar, 100 μg ml−1 rifampicin). (B) Establishment of HRO-C48 in the rhizosphere after 15, 30, 45 and 60 days depending on seed treatment. Significant differences (P < 0.05) were determined using Fisher’s protected least significance difference (LSD), the same letter above bars indicate that there were no significant differences. Error bars represent confidence interval (P = 0.05)
In a second trial, the influence of different seed treatments on the colonization of S. plymuthica HRO-C48Rif r in the oilseed rape rhizosphere was examined. Different strategies, which were used to apply Serratia cells on/in rape seeds, are shown in Fig. 1. To analyze the impact of the application method, for all treatments identical initial bacterial cell densities of log10 6.0 CFU seed−1 were applied. After 15 days of plant growth, a slightly higher number of S. plymuthica HRO-C48Rif r cells had established in the rhizosphere using bio-priming (log10 4.75 CFU g−1 root fresh mass) than using pelleting (log10 4.23) and film coating (log10 4.14), respectively (Fig. 2B). In the course of time the abundances decreased successively. After 30 days on average log10 4.09, after 45 days log10 3.55 and after 60 days log10 3.24 CFU per gram root fresh mass could be re-isolated. The root-associated cell numbers were indistinguishable regarding the inoculation method.
Evaluation of disease suppression effect
The ability of Serratia cells to reduce disease incidence caused by V. longisporum was analyzed in greenhouse trials using artificially infested soil. All plants included in the experiment developed typical symptoms of the Verticillium wilt. In general, all Serratia treated plants had a significantly lower disease severity expressed by AUDPC compared to the untreated control (Fig. 3). However, different application methods resulted in different biocontrol efficiencies. Whereas film coating led in a disease suppression of 5.2%, plants treated by pelleting and bio-priming showed a higher reduction of disease severity of 13.4% and 14.3%, respectively.
Influence of different seed treatments with and without S. plymuthica HRO-C48 on the development of Verticillium wilt expressed by area under disease process curve in comparison to the untreated control. Oilseed rape seeds were treated with S. plymuthica HRO-C48 cells at concentrations of log10 6.0. Significant differences (P < 0.05) were determined using Fisher’s protected least significance difference (LSD), the same letter above bars indicate that there were no significant differences. Error bars represent confidence interval (P = 0.05)
Stability of S. plymuthica HRO-C48 in formulations
The survival of S. plymuthica cells in dependence on the seed treatment technique was determined over a period of 30 days at storage temperatures of 4°C and 20°C in a climate chamber (Fig. 4). At the beginning of the experiment, about log10 7.0 CFU seed−1 were re-isolated from the seed surface using pelleting and film coating. In contrast, after bio-priming less cells (log10 6.0 CFU seed−1) of HRO-C48 were found to be associated with bio-primed seeds. Importantly, bacteria in infiltrated seeds were located inside the seed whereas using the other treatments, cells were attached to the seed surface. After storage of 30 days at 4°C bacteria on pelleted seeds showed the highest survival rate at log10 5.9 ± 0.07 seed−1. From film-coated seeds log10 4.2 ± 0.15 CFU seed−1, and from primed seeds log10 4.8 ± 0.18 CFU seed−1 could be re-isolated. Cells of S. plymuthica localized at the surface of seeds, showed less stability during storage at 20°C than cells in infiltrated seeds. After 30 days the following plate counts of Serratia cells associated with differently treated seeds were determined: log10 2.6 ± 0.18 using pelleting, log10 2.5 ± 0.64 using film coating, and log10 4.7 ± 0.29 CFU seed−1 applied by bio-priming. For the latter, statistically significant more Serratia cells survived the storage than using the other treatments.
Stability of S. plymuthica HRO-C48 applied to oilseed rape seeds using different treatments at concentrations of log10 6.0 (seed priming), and log10 7.0 CFU seed−1 (pelleting, film coating). Treated seeds were stored at 4°C and 20°C. Bacteria were re-isolated after 30 days on nutrient agar. Error bars represent confidence interval (P = 0.05)
Optimization of bio-priming procedure
Bio-priming procedure has to be aligned with the time course of seed germination. In preliminary experiments we found that the germination behavior depends on the cultivar and the quality of seeds (data not shown). The time course of germination is presented in Fig. 5. Seeds went through the phase of imbibition (phase I) in the first 4 h, the activation phase (phase II) was located between 4 h and 16 h, and the last phase of germination (phase III) began after 16 h. According to the three phases of germination the development of the cell numbers of S. plymuthica HRO-C48 during the process of bio-priming was determined. An abundance of log10 3.4 ± 0.40 CFU seed−1 were found inside the seed after 4 h. Despite water uptake and imbibition period was completed, a higher level of bacteria was achieved after 12 h (log10 6.2 ± 0.26 CFU seed−1), suggesting that bacteria either actively migrate into the seed or multiply within the seed. No further increase of cell counts was observed after 20 h (log10 6.3 ± 0.21 CFU seed−1).
Discussion
During the last decade Verticillium wilt in oilseed rape has become a serious problem worldwide. Due to the economic importance of yield losses caused by the pathogen, the objective of our study was to find biological solutions to suppress the fungus. A potential candidate for this purpose is the rhizobacterium S. plymuthica HRO-C48, which was successfully employed to suppress V. dahliae in strawberries. Altogether, it was possible to apply Serratia cells to oilseed rape seeds resulting in a colonization of rape roots under greenhouse conditions. In addition, suppressive effects on V. dahliae could be established. Applying the BCA directly to seed is an attractive proposition because the bacterium will be at the right place with the beginning of seed germination (Lumsden et al. 1995). Overall, three seed treatment methods to apply S. plymuthica HRO-C48 to seeds were selected for assessment, and were evaluated according to the following industrial and environmental demands posited by Rhodes (1993): The inoculation process must be easily practicable and the biological control agent must endure various outside influences during formulation, storage, transportation and sowing procedure without dying or loosing effectiveness. Additionally, the utilized seed treatment procedure do not have a negative impact and has to be non-impact compatible with existing technologies, as well as being feasible for large-scale utilization (Powell 1993).
Pelleting is a commonplace method to apply beneficial microorganisms to seeds (Burgues 1998). The procedure is simple and can be easily integrated into conventional seed treatment processes and machinery. A lot of experience exists for the technique of pelleting as well as for utilized materials. The possibility of applying additives to improve the stability of bacterial cells is another advantage of this procedure. After pelleting, seeds have to be re-dried avoiding germination of the seed during intermediate storage, which would have an adverse effect on the seed quality. Thus, the bacteria must survive a period of low water activity (Fravel et al. 1998). This might be one reason for the problematic storage of pelleted seeds at 20°C. In contrast, at 4°C the survival rate of Serratia cells in the pellet is significant higher but storage at this temperature is rarely found in agricultural practices. Furthermore, pelleted seeds are quite larger than the untreated ones. Therefore, it is may be necessary to adapt the available drilling techniques. The efficiency of HRO-C48 applied by pelleting to suppress Verticillium wilt was shown. Plant health was statistically significantly enhanced. Altogether, the inoculation of rape seeds with S. plymuthica by pelleting is reliable and effective for oilseed rape under greenhouse conditions. Because of the low cell stability at moderate temperatures inoculated seeds should be stored under refrigerated conditions, which is problematic and/or expensive. However, this procedure can be further optimized for example by adding protective compounds. One promising group are osmoprotectans, which are able to protect bacterial cells against changing salinities and dryness. S. plymuthica HRO-C48 endogenously accumulates two different osmoprotective substances, viz. proline and glycine betain (unpublished results). To employ these substances, two ways are possible: (i) to enhance the content of endogenous osmoprotectans by cultivating under high saline conditions or (ii) to add exogenous osmolytes such as trehalose or glucosylglycerol.
The second seed treatment procedure investigated was film coating, in which Serratia cells were lyophilized directly on the seed coat, resulting in a thin layer surrounding the seed. Lyophilization is a method for the stabilization of bacterial cells, and is commonly used in both research and industry (Souzo 1992). After lyophilization bacteria were integrated into a very thin layer around the seed, which is comparable in size with an untreated seed. No new machines or strategies have to be developed if film-coated seeds will be used for following conventional seed treatments. One major disadvantage is that the actual procedure of lyophilization is costly and requires special equipment. In comparison to the other treatments, HRO-C48 did not show a higher survival rate on the seed surface. In addition, a negative influence was found on the disease suppression capacity of S. plymuthica. This is in agreement with Stephan et al. (2006), who have reported that the ability of Pseudomonas fluorescens strain Pf 153 to control Botrytis cinerea in field bean plants was lacking after the bacteria were lyophilized in presence of sucrose. Lyophilized and re-activated bacterial cells could have lost their activity because of unfavorable rehydration conditions in the planting substrate. The rehydration conditions are proposed to be critical for the physiological state of the revitalized cells (Costa et al. 2000). Altogether, this method was not useful to apply Serratia to rape seeds.
The third procedure, which was used to apply Serratia cells to seeds, was bio-priming. Seed priming is a well established procedure especially for vegetables, which is used to get a faster and more uniform germination of seeds (Gray 1994). In addition, bio-priming was used to apply BCAs to seeds for biological control of Pythium ultimum preemergence damping-off in sweet corn (Callan et al. 1990). This procedure is practicable and implementable into commercial seed production. However, the effect of bio-priming depends on the cultivar as well as on the quality of seeds. To prevent loss in germination capacity, preliminary experiments were necessary to optimize bio-priming process. To obtain an optimal cell density inside the seed, the procedure should be stopped in the middle of the activation phase (phase II of seed germination according to Bradford 1995), as shown in our study. At this time, water uptake is completed and the maximal number of bacterial cells have been established in the interior of the seed. In our experiments, the survival of Serratia cells inside the rape seeds was excellent. At 4°C, the stability was comparable to pelleting but at 20°C it was higher than for the other treatments. In contrast to other treatments, using bio-priming bacteria did not only establish on the surface, they also invade the seeds. Inside the seeds bacteria are protected against unfavorable abiotic and biotic conditions. For example, seeds usually coated with chemical pesticides, which may interact negatively with the biological inoculant. By using bio-priming bacterial cells are physically separated from these deleterious agents. In particular, during our study we observed that the insecticide Chinook (Bayer, Dormagen, Germany), which is obligatory used for rape seeds, had a lethal influence on HRO-C48, when it is in the same formulation (data not shown). Interestingly, the population density of HRO-C48 in the rhizosphere of bio-primed plants was slightly higher after 15 days compared to the other treatments. By the use of bio-priming the bacteria get in intimate contact with the seed embryo resulting in immediate attachment to the radicals during germination. Because Verticillium can infect oilseed rape plants throughout the vegetation period a high cell density from the point of seedling emergence is desirable. Concluding, bio-priming is an excellent and innovative method to apply Serratia to rape seeds.
All of our studies were carried out under greenhouse conditions. In general, the pathogen V. longisporum is difficult to handle because the fungus grows very slowly. In the field, Verticillium symptoms occur some days or weeks before harvesting, although the infection by the fungus can take place anytime in the entire vegetation period (Daebeler et al. 1988). In Northern Europe, rape seeds are sown in August and are harvested in June/July of the following year. This means that field trials to evaluate pathogen suppressing strategies would take about 11 month. For our objective, a rapid greenhouse assay to investigate all parameters was required. For these reasons, in our experiments we applied the pathogen at inoculum densities, which were much higher than those found in natural soils (Termorshuizen et al. 1998). Consequentially, the results of these greenhouse trials were used to compare the seed treatments and do not reflect the real efficiency of S. plymuthica under field conditions. In the next step of our investigations the results will be verified in soils naturally infested with V. longisporum.
In summary, it was possible to apply Serratia cells to oilseed rape seeds resulting in establishment of effective bacterial populations in the rhizosphere. In addition, the present study demonstrates the suppressive effects of the BCA on V. longisporum under greenhouse conditions. According to the assessment of certain criteria the application of bio-priming was evaluated as the most suitable method for inoculating oilseed rape seeds with HRO-C48. Pelleting and film coating was proved to be unfeasible for the application of S. plymuthica to seeds.
References
Barbara D, Clewes E (2003) Plant pathogenic Verticillium species: how many of them are there? Mol Plant Pathol 4:297–305
Berg G, Kurze S, Dahl R (1999) Isolated rhizobacteria for treatment of phytopathogenic fungal diseases. Eur Patent Nr 98124694.5
Berg G (2000) Diversity of antifungal and plant-associated Serratia plymuthica strains. J Appl Microbiol 88:952–960
Berg G, Fritze A, Roskot N, Smalla K (2001) Evaluation of potential biocontrol rhizobacteria from different host plants of Verticillium dahliae Kleb. J Appl Microbiol 156:75–82
Bradford KJ (1995) Water relations in seed germination. In: Kigel J, Galili G (eds) Seed development and germination. Marcel Dekker Inc., New York, pp 351–396
Burgues HD (1998) Formulation of microbial pesticides. Beneficial microorganisms, nematodes and seed treatments. Kluwer Academic Publisher, London
Callan NW, Marthre DE, Miller JB (1990) Bio-priming seed treatment for biological control of Pythium ultimum preemergence damping-off in sh-2 sweet corn. Plant Dis 74:368–372
Conn KL, Lazarovits G (2000) Soil factors influencing the efficacy of liquid swine manure added to soil to kill Verticillium dahliae. Can J Plant Pathol 21:400–406
Costa E, Usall J, Teixido N, Garcia N, Vinas I (2000) Effect of protective agents, rehydration media and initial cell concentration on viability of Pantoea agglomerans strain CPA-2 subjected to freeze-drying. J Appl Microbiol 89:793–800
Daebeler F, Amelung D, Zeise K (1988) Verticillium-welke an winterraps - auftreten und bedeutung. Nachrichtenblatt Pflanzenschutz DDR 42:71–73
Debode J, Spiessens K, De Rooster L, Hofte M (2002) Verticillium wilt of cauliflower in Belgium. Meded Rijksuniv Gent Fak Landbouwkd Toegep Biol Wet 67(2):241–249
Emmert EAB, Handelsman J (1999) Biocontrol of plant disease: a (Gram+) positive perspective. FEMS Microbiol Lett 171:1–9
Fahleson J, Hu Q, Dixelius C (2004) Phylogenetic analysis of Verticillium species based on nuclear and mitochondrial sequences. Arch Microbiol 181:435–442
Frankowski J, Lorito M, Schmid R, Berg G, Bahl H (2001) Purification and properties of two chitinolytic enzymes of Serratia plymuthica HRO-C48. Arch Microbiol 176:421–426
Fravel DR, Connik WJ, Lewis JA (1998) Formulation of microorganisms to control plant diseases. In: Burgues HD (ed) Formulation of microbial pesticides. Beneficial microorganisms, nematodes and seed treatments. Kluwer Academic Publisher, London, pp 187–202
Graner G, Persson P, Meijer J, Alstrom S (2003) A study on microbial diversity in different cultivars of Brassica napus in relation to its wilt pathogen, Verticillium longisporum. FEMS Microbiol Lett 29:269–276
Gray D (1994) Large-scale seed priming techniques and their integration with crop protection treatments. In: Martin T (ed) Seed treatment: progress and prospects, monograph 57. British Crop Protection Council, Farnham, pp 353–362
Karapapa VK, Bainbridge BW, Heale JB (1997) Morphological and molecular characterization of Verticillium longisporum comb. nov., pathogenic to oilseed rape. Mycol Res 101:1281–1294
Kalbe C, Marten P, Berg G (1996) Members of the genus Serratia as beneficial rhizobacteria of oilseed rape. Microbiol Res 151:4433–4400
Kurze S, Dahl R, Bahl H, Berg G (2001a) Biological control of soil-borne pathogens in strawberry by Serratia plymuthica HRO-C48. Plant Dis 85:529–534
Kurze S, Sauerbrunn N, Bahl H, Dahl R, Berg G (2001b) Effects of antagonistic rhizobacteria on plant health, yield, and the bacterial rhizosphere community of strawberry. IOBC Bulletin 24:117–120
Lumsden RD, Lewis JA, Fravel DR (1995) Formulation and delivery of biocontrol agents for use against soilborne plant pathogens. In: Hall FR, Barry JW (eds) Biorational pest control agents. Formulation and delivery, Washington, pp 166–182
Martin FN (2003) Development of alternative strategies for management of soilborne pathogens currently controlled with methyl bromide. Ann Rev Phytopathol 41:325–350
Mercado-Blanco J, Rodríguez-Jurado D, Hervás A, Jiménez-Díaz RM (2004) Suppression of Verticillium wilt in olive planting stocks by root-associated fluorescent P. seudomonas sp. Biol Control 30:474–486
Messner R, Schweigkofler W, Schweigkofler M, Berg G, Prillinger H (1996) Molecular characterization of the plant pathogen Verticillium dahliae Kleb. using RAPD-PCR and sequencing of the 18S rRNA-gene. J Phytopathol 144:347–354
Powell KA (1993) The commercial exploitation of microorganisms in agriculture. In: Jones DG (ed) Exploitation of microorganisms. Chapman and Hall, New York, pp 441–459
Rhodes DJ (1993) Formulation of biological control agents. In: Jones DG (ed) Exploitation of microorganisms. Chapman & Hall, London, pp 411–439
Scherwinski K, Wolf A, Berg G (2007) Assessing the risk of biological control agents on the indigenous microbial communities: Serratia plymuthica HRO-C48 and Streptomyces sp. HRO-71 as model bacteria. Biol Control 52:87–112
Souzo H (1992) Freeze-drying of microorganisms. Encyclopedia of microbiology, vol 2. Academic Press, New York
Stark C (1961) Das auftreten der Verticillium-tracheomykose in hamburger gartenbaukulturen. Gartenbauwiss 26:493–528
Stephan D, Bisutti IL, da Silva A-P (2006) Optimisation of the freeze drying process of Pseudomonas fluorescens strain CHA0 and Pf 153. IXth Meeting of the IOBC/WPRS working group “Integrated Control of Fungal and Bacterial Plant Pathogens”, Spa, Belgium, 6-10 September 2006
Steventon LA, Fahleson J, Hu Q, Dixelius C (2002) Identification of the causal agent of Verticillium wilt of winter oilseed rape in Sweden as Verticillium longisporum. Mycol Res 106:570–578
Subbarao KV, Hubbard JC, Koike ST (1999) Evaluation of broccoli residue incorporation into field soil for Verticillium wilt control in cauliflower. Plant Dis 83:124–129
Termorshuizen AJ, Davis JR, Gort G, Harris DC, Huisman OC, Lazarovits G, Locke T, Melero Vara JM, Mol L, Papalomatas EJ, Platt HW, Powelson M, Rouse DI, Rowe RC, Tsor L (1998) Interlaboratory comparison of methods to quantify microsclerotia of Verticillium dahliae in soil. Appl Environ Microbiol 64:3846–3853
Tjamos EC, Rowe RC, Heale JB, Fravel DR (2000) Advances in Verticillium research and disease management. APS Press. The American Phytopathological Society, St. Paul, Minnesota
Tjamos EC, Tsitsigiannis DI, Tjamos SE, Antoniou P, Katinakis P (2004) Selection and screening of endorhizosphere bacteria from solarised soils as biocontrol agents against Verticillium dahliae of solanaceous hosts. Eur J Plant Pathol 110:35–44
Weller DM (1988) Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu Rev Phytopathol 26:379–407
Whipps JM (1997) Ecological considerations involved in commercial development of biological control agents for soil-borne diseases. In: Van Elsas JD, Trevors JT, Wellington EMH (eds) Modern soil microbiology. Marcel Dekker, Inc., New York, Basel, Hongkong, pp 525–545
Whipps J (2001) Microbial interactions and biocontrol in the rhizosphere. J Exp Bot 52:487–511
Zeise K (1992) Screening for resistance to Verticillium dahliae Kleb. on oilseed rape (Brassica napus var. oleifera METZGER) under greenhouse conditions. Nachrichtenbl Deut Pflanzenschutzdienst 44:125–128
Zeise K, von Tiedemann A (2001) Morphological and physiological differentiation among vegetative compatibility groups of Verticillium dahliae in relation to Verticillium longisporum. J Phytopathol 149:469–475
Acknowledgements
We thank Hella Goschke for valuable technical assistance. We are very grateful to the Norddeutsche Pflanzenzucht Hans-Georg Lembke KG (Hohenlieth, Germany) and to the German Federal Environmental Foundation for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müller, H., Berg, G. Impact of formulation procedures on the effect of the biocontrol agent Serratia plymuthica HRO-C48 on Verticillium wilt in oilseed rape. BioControl 53, 905–916 (2008). https://doi.org/10.1007/s10526-007-9111-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-007-9111-3